Toxicity measurements in concentrated water samples - Rivm
Toxicity measurements in concentrated water samples - Rivm
Toxicity measurements in concentrated water samples - Rivm
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
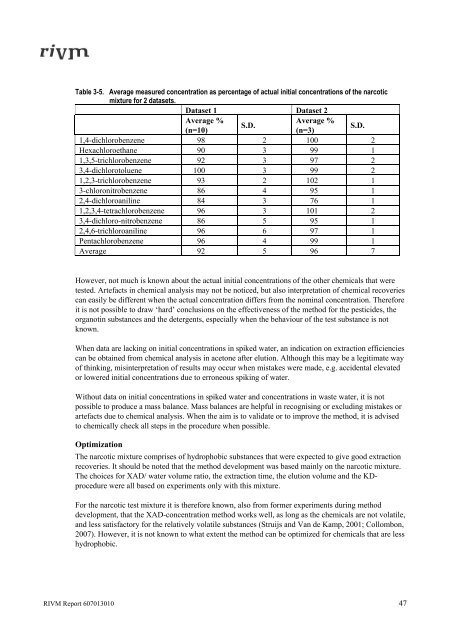
Table 3-5. Average measured concentration as percentage of actual <strong>in</strong>itial concentrations of the narcotic<br />
mixture for 2 datasets.<br />
Dataset 1 Dataset 2<br />
Average %<br />
(n=10)<br />
S.D.<br />
Average %<br />
(n=3)<br />
S.D.<br />
1,4-dichlorobenzene 98 2 100 2<br />
Hexachloroethane 90 3 99 1<br />
1,3,5-trichlorobenzene 92 3 97 2<br />
3,4-dichlorotoluene 100 3 99 2<br />
1,2,3-trichlorobenzene 93 2 102 1<br />
3-chloronitrobenzene 86 4 95 1<br />
2,4-dichloroanil<strong>in</strong>e 84 3 76 1<br />
1,2,3,4-tetrachlorobenzene 96 3 101 2<br />
3,4-dichloro-nitrobenzene 86 5 95 1<br />
2,4,6-trichloroanil<strong>in</strong>e 96 6 97 1<br />
Pentachlorobenzene 96 4 99 1<br />
Average 92 5 96 7<br />
However, not much is known about the actual <strong>in</strong>itial concentrations of the other chemicals that were<br />
tested. Artefacts <strong>in</strong> chemical analysis may not be noticed, but also <strong>in</strong>terpretation of chemical recoveries<br />
can easily be different when the actual concentration differs from the nom<strong>in</strong>al concentration. Therefore<br />
it is not possible to draw ‘hard’ conclusions on the effectiveness of the method for the pesticides, the<br />
organot<strong>in</strong> substances and the detergents, especially when the behaviour of the test substance is not<br />
known.<br />
When data are lack<strong>in</strong>g on <strong>in</strong>itial concentrations <strong>in</strong> spiked <strong>water</strong>, an <strong>in</strong>dication on extraction efficiencies<br />
can be obta<strong>in</strong>ed from chemical analysis <strong>in</strong> acetone after elution. Although this may be a legitimate way<br />
of th<strong>in</strong>k<strong>in</strong>g, mis<strong>in</strong>terpretation of results may occur when mistakes were made, e.g. accidental elevated<br />
or lowered <strong>in</strong>itial concentrations due to erroneous spik<strong>in</strong>g of <strong>water</strong>.<br />
Without data on <strong>in</strong>itial concentrations <strong>in</strong> spiked <strong>water</strong> and concentrations <strong>in</strong> waste <strong>water</strong>, it is not<br />
possible to produce a mass balance. Mass balances are helpful <strong>in</strong> recognis<strong>in</strong>g or exclud<strong>in</strong>g mistakes or<br />
artefacts due to chemical analysis. When the aim is to validate or to improve the method, it is advised<br />
to chemically check all steps <strong>in</strong> the procedure when possible.<br />
Optimization<br />
The narcotic mixture comprises of hydrophobic substances that were expected to give good extraction<br />
recoveries. It should be noted that the method development was based ma<strong>in</strong>ly on the narcotic mixture.<br />
The choices for XAD/ <strong>water</strong> volume ratio, the extraction time, the elution volume and the KDprocedure<br />
were all based on experiments only with this mixture.<br />
For the narcotic test mixture it is therefore known, also from former experiments dur<strong>in</strong>g method<br />
development, that the XAD-concentration method works well, as long as the chemicals are not volatile,<br />
and less satisfactory for the relatively volatile substances (Struijs and Van de Kamp, 2001; Collombon,<br />
2007). However, it is not known to what extent the method can be optimized for chemicals that are less<br />
hydrophobic.<br />
RIVM Report 607013010 47