Dietary supplements: FDA may have opportunities to expand its use
Dietary supplements: FDA may have opportunities to expand its use
Dietary supplements: FDA may have opportunities to expand its use
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
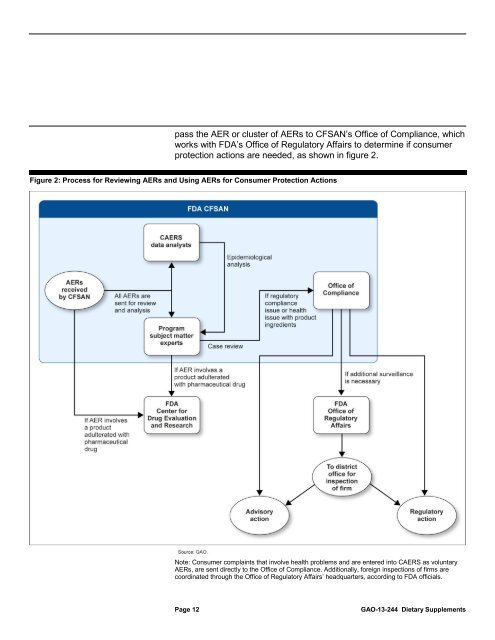
pass the AER or cluster of AERs <strong>to</strong> CFSAN’s Office of Compliance, which<br />
works with <strong>FDA</strong>’s Office of Regula<strong>to</strong>ry Affairs <strong>to</strong> determine if consumer<br />
protection actions are needed, as shown in figure 2.<br />
Figure 2: Process for Reviewing AERs and Using AERs for Consumer Protection Actions<br />
Note: Consumer complaints that involve health problems and are entered in<strong>to</strong> CAERS as voluntary<br />
AERs, are sent directly <strong>to</strong> the Office of Compliance. Additionally, foreign inspections of firms are<br />
coordinated through the Office of Regula<strong>to</strong>ry Affairs’ headquarters, according <strong>to</strong> <strong>FDA</strong> officials.<br />
Page 12<br />
GAO-13-244 <strong>Dietary</strong> Supplements