Dietary supplements: FDA may have opportunities to expand its use
Dietary supplements: FDA may have opportunities to expand its use
Dietary supplements: FDA may have opportunities to expand its use
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
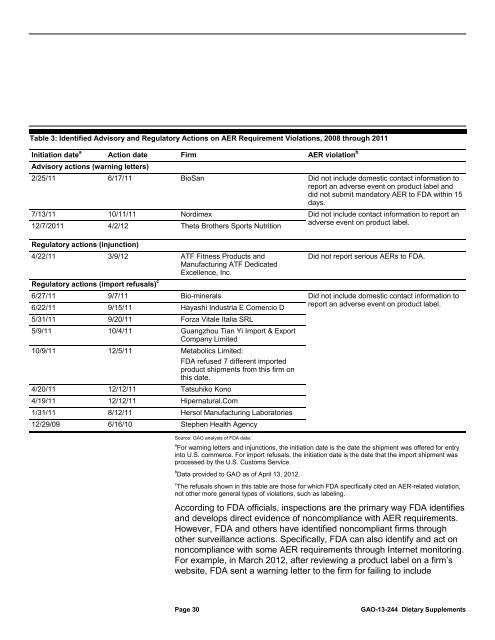
Table 3: Identified Advisory and Regula<strong>to</strong>ry Actions on AER Requirement Violations, 2008 through 2011<br />
Initiation date<br />
a<br />
Action date<br />
Advisory actions (warning letters)<br />
Firm<br />
AER violation b<br />
2/25/11 6/17/11 BioSan Did not include domestic contact information <strong>to</strong><br />
report an adverse event on product label and<br />
did not submit manda<strong>to</strong>ry AER <strong>to</strong> <strong>FDA</strong> within 15<br />
days.<br />
7/13/11 10/11/11 Nordimex Did not include contact information <strong>to</strong> report an<br />
12/7/2011 4/2/12 Theta Brothers Sports Nutrition<br />
adverse event on product label.<br />
Regula<strong>to</strong>ry actions (injunction)<br />
4/22/11 3/9/12 ATF Fitness Products and<br />
Did not report serious AERs <strong>to</strong> <strong>FDA</strong>.<br />
Manufacturing ATF Dedicated<br />
Excellence, Inc.<br />
c<br />
Regula<strong>to</strong>ry actions (import refusals)<br />
6/27/11<br />
9/7/11 Bio-minerals Did not include domestic contact information <strong>to</strong><br />
6/22/11 9/15/11 Hayashi Industria E Comercio D<br />
report an adverse event on product label.<br />
5/31/11 9/20/11 Forza Vitale Italia SRL<br />
5/9/11 10/4/11 Guangzhou Tian Yi Import & Export<br />
Company Limited<br />
10/9/11 12/5/11 Metabolics Limited:<br />
<strong>FDA</strong> ref<strong>use</strong>d 7 different imported<br />
product shipments from this firm on<br />
this date.<br />
4/20/11 12/12/11 Tatsuhiko Kono<br />
4/19/11 12/12/11 Hipernatural.Com<br />
1/31/11 8/12/11 Hersol Manufacturing Labora<strong>to</strong>ries<br />
12/29/09 6/16/10 Stephen Health Agency<br />
Source: GAO analysis of <strong>FDA</strong> data.<br />
a For warning letters and injunctions, the initiation date is the date the shipment was offered for entry<br />
in<strong>to</strong> U.S. commerce. For import refusals, the initiation date is the date that the import shipment was<br />
processed by the U.S. Cus<strong>to</strong>ms Service.<br />
b Data provided <strong>to</strong> GAO as of April 13, 2012.<br />
c<br />
The refusals shown in this table are those for which <strong>FDA</strong> specifically cited an AER-related violation,<br />
not other more general types of violations, such as labeling.<br />
According <strong>to</strong> <strong>FDA</strong> officials, inspections are the primary way <strong>FDA</strong> identifies<br />
and develops direct evidence of noncompliance with AER requirements.<br />
However, <strong>FDA</strong> and others <strong>have</strong> identified noncompliant firms through<br />
other surveillance actions. Specifically, <strong>FDA</strong> can also identify and act on<br />
noncompliance with some AER requirements through Internet moni<strong>to</strong>ring.<br />
For example, in March 2012, after reviewing a product label on a firm’s<br />
website, <strong>FDA</strong> sent a warning letter <strong>to</strong> the firm for failing <strong>to</strong> include<br />
Page 30<br />
GAO-13-244 <strong>Dietary</strong> Supplements