Dietary supplements: FDA may have opportunities to expand its use
Dietary supplements: FDA may have opportunities to expand its use
Dietary supplements: FDA may have opportunities to expand its use
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Appendix II: Data on <strong>FDA</strong>’s Consumer<br />
Protection Actions Related <strong>to</strong> <strong>Dietary</strong><br />
Supplements<br />
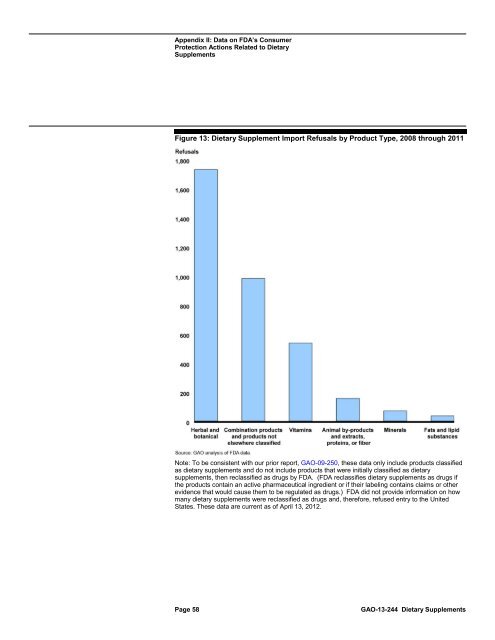
Figure 13: <strong>Dietary</strong> Supplement Import Refusals by Product Type, 2008 through 2011<br />
Note: To be consistent with our prior report, GAO-09-250, these data only include products classified<br />
as dietary <strong>supplements</strong> and do not include products that were initially classified as dietary<br />
<strong>supplements</strong>, then reclassified as drugs by <strong>FDA</strong>. (<strong>FDA</strong> reclassifies dietary <strong>supplements</strong> as drugs if<br />
the products contain an active pharmaceutical ingredient or if their labeling contains claims or other<br />
evidence that would ca<strong>use</strong> them <strong>to</strong> be regulated as drugs.) <strong>FDA</strong> did not provide information on how<br />
many dietary <strong>supplements</strong> were reclassified as drugs and, therefore, ref<strong>use</strong>d entry <strong>to</strong> the United<br />
States. These data are current as of April 13, 2012.<br />
Page 58<br />
GAO-13-244 <strong>Dietary</strong> Supplements