Dietary supplements: FDA may have opportunities to expand its use
Dietary supplements: FDA may have opportunities to expand its use
Dietary supplements: FDA may have opportunities to expand its use
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
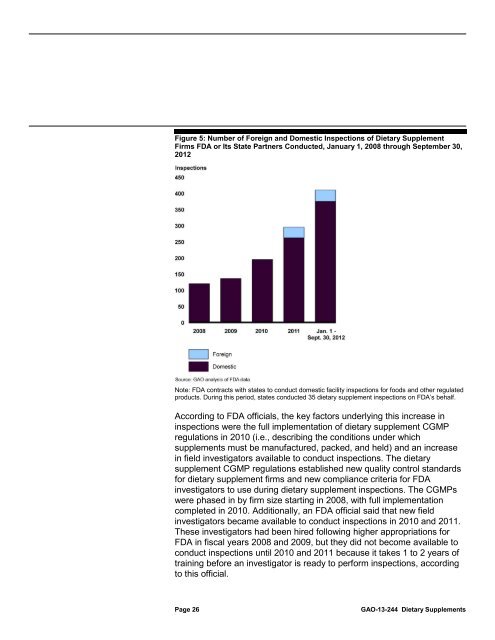
Figure 5: Number of Foreign and Domestic Inspections of <strong>Dietary</strong> Supplement<br />
Firms <strong>FDA</strong> or Its State Partners Conducted, January 1, 2008 through September 30,<br />
2012<br />
Note: <strong>FDA</strong> contracts with states <strong>to</strong> conduct domestic facility inspections for foods and other regulated<br />
products. During this period, states conducted 35 dietary supplement inspections on <strong>FDA</strong>’s behalf.<br />
According <strong>to</strong> <strong>FDA</strong> officials, the key fac<strong>to</strong>rs underlying this increase in<br />
inspections were the full implementation of dietary supplement CGMP<br />
regulations in 2010 (i.e., describing the conditions under which<br />
<strong>supplements</strong> must be manufactured, packed, and held) and an increase<br />
in field investiga<strong>to</strong>rs available <strong>to</strong> conduct inspections. The dietary<br />
supplement CGMP regulations established new quality control standards<br />
for dietary supplement firms and new compliance criteria for <strong>FDA</strong><br />
investiga<strong>to</strong>rs <strong>to</strong> <strong>use</strong> during dietary supplement inspections. The CGMPs<br />
were phased in by firm size starting in 2008, with full implementation<br />
completed in 2010. Additionally, an <strong>FDA</strong> official said that new field<br />
investiga<strong>to</strong>rs became available <strong>to</strong> conduct inspections in 2010 and 2011.<br />
These investiga<strong>to</strong>rs had been hired following higher appropriations for<br />
<strong>FDA</strong> in fiscal years 2008 and 2009, but they did not become available <strong>to</strong><br />
conduct inspections until 2010 and 2011 beca<strong>use</strong> it takes 1 <strong>to</strong> 2 years of<br />
training before an investiga<strong>to</strong>r is ready <strong>to</strong> perform inspections, according<br />
<strong>to</strong> this official.<br />
Page 26<br />
GAO-13-244 <strong>Dietary</strong> Supplements