Dietary supplements: FDA may have opportunities to expand its use
Dietary supplements: FDA may have opportunities to expand its use
Dietary supplements: FDA may have opportunities to expand its use
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Appendix II: Data on <strong>FDA</strong>’s Consumer<br />
Protection Actions Related <strong>to</strong> <strong>Dietary</strong><br />
Supplements<br />
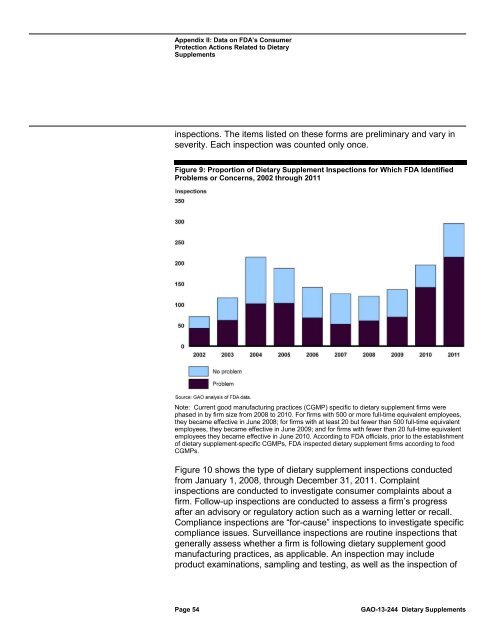
inspections. The items listed on these forms are preliminary and vary in<br />
severity. Each inspection was counted only once.<br />
Figure 9: Proportion of <strong>Dietary</strong> Supplement Inspections for Which <strong>FDA</strong> Identified<br />
Problems or Concerns, 2002 through 2011<br />
Note: Current good manufacturing practices (CGMP) specific <strong>to</strong> dietary supplement firms were<br />
phased in by firm size from 2008 <strong>to</strong> 2010. For firms with 500 or more full-time equivalent employees,<br />
they became effective in June 2008; for firms with at least 20 but fewer than 500 full-time equivalent<br />
employees, they became effective in June 2009; and for firms with fewer than 20 full-time equivalent<br />
employees they became effective in June 2010. According <strong>to</strong> <strong>FDA</strong> officials, prior <strong>to</strong> the establishment<br />
of dietary supplement-specific CGMPs, <strong>FDA</strong> inspected dietary supplement firms according <strong>to</strong> food<br />
CGMPs.<br />
Figure 10 shows the type of dietary supplement inspections conducted<br />
from January 1, 2008, through December 31, 2011. Complaint<br />
inspections are conducted <strong>to</strong> investigate consumer complaints about a<br />
firm. Follow-up inspections are conducted <strong>to</strong> assess a firm’s progress<br />
after an advisory or regula<strong>to</strong>ry action such as a warning letter or recall.<br />
Compliance inspections are “for-ca<strong>use</strong>” inspections <strong>to</strong> investigate specific<br />
compliance issues. Surveillance inspections are routine inspections that<br />
generally assess whether a firm is following dietary supplement good<br />
manufacturing practices, as applicable. An inspection <strong>may</strong> include<br />
product examinations, sampling and testing, as well as the inspection of<br />
Page 54<br />
GAO-13-244 <strong>Dietary</strong> Supplements