Dietary supplements: FDA may have opportunities to expand its use
Dietary supplements: FDA may have opportunities to expand its use
Dietary supplements: FDA may have opportunities to expand its use
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Appendix II: Data on <strong>FDA</strong>’s Consumer<br />
Protection Actions Related <strong>to</strong> <strong>Dietary</strong><br />
Supplements<br />
the firm and facility involved with dietary <strong>supplements</strong>, according <strong>to</strong> <strong>FDA</strong><br />
officials.<br />
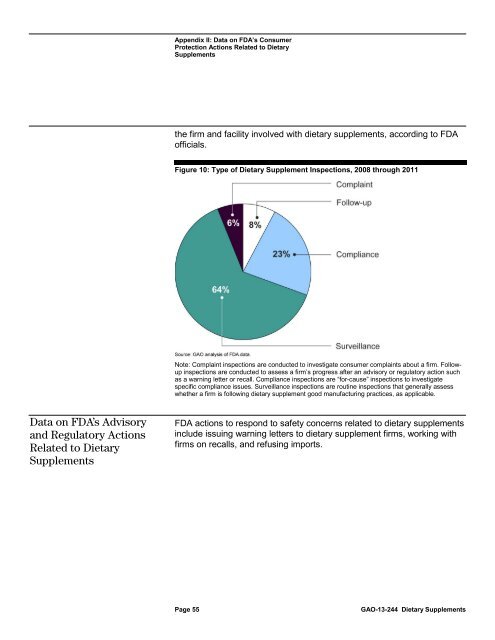
Figure 10: Type of <strong>Dietary</strong> Supplement Inspections, 2008 through 2011<br />
Note: Complaint inspections are conducted <strong>to</strong> investigate consumer complaints about a firm. Followup<br />
inspections are conducted <strong>to</strong> assess a firm’s progress after an advisory or regula<strong>to</strong>ry action such<br />
as a warning letter or recall. Compliance inspections are “for-ca<strong>use</strong>” inspections <strong>to</strong> investigate<br />
specific compliance issues. Surveillance inspections are routine inspections that generally assess<br />
whether a firm is following dietary supplement good manufacturing practices, as applicable.<br />
Data on <strong>FDA</strong>’s Advisory<br />
and Regula<strong>to</strong>ry Actions<br />
Related <strong>to</strong> <strong>Dietary</strong><br />
Supplements<br />
<strong>FDA</strong> actions <strong>to</strong> respond <strong>to</strong> safety concerns related <strong>to</strong> dietary <strong>supplements</strong><br />
include issuing warning letters <strong>to</strong> dietary supplement firms, working with<br />
firms on recalls, and refusing imports.<br />
Page 55<br />
GAO-13-244 <strong>Dietary</strong> Supplements