Dietary supplements: FDA may have opportunities to expand its use
Dietary supplements: FDA may have opportunities to expand its use
Dietary supplements: FDA may have opportunities to expand its use
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>to</strong> provide information on AERs <strong>FDA</strong> received from January 1, 2003,<br />
through September 30, 2012. 24<br />
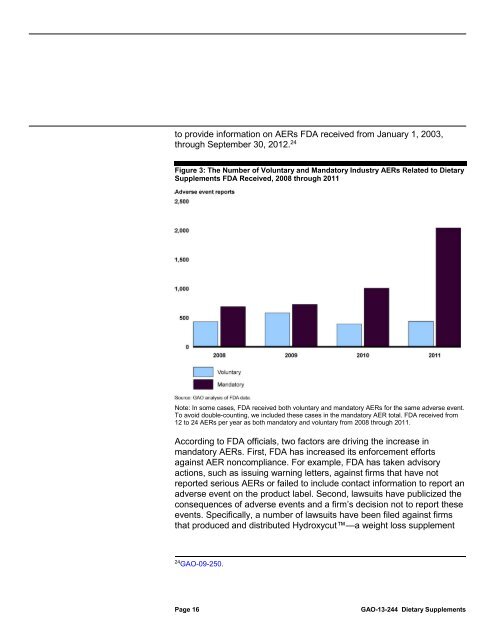
Figure 3: The Number of Voluntary and Manda<strong>to</strong>ry Industry AERs Related <strong>to</strong> <strong>Dietary</strong><br />
Supplements <strong>FDA</strong> Received, 2008 through 2011<br />
Note: In some cases, <strong>FDA</strong> received both voluntary and manda<strong>to</strong>ry AERs for the same adverse event.<br />
To avoid double-counting, we included these cases in the manda<strong>to</strong>ry AER <strong>to</strong>tal. <strong>FDA</strong> received from<br />
12 <strong>to</strong> 24 AERs per year as both manda<strong>to</strong>ry and voluntary from 2008 through 2011.<br />
According <strong>to</strong> <strong>FDA</strong> officials, two fac<strong>to</strong>rs are driving the increase in<br />
manda<strong>to</strong>ry AERs. First, <strong>FDA</strong> has increased <strong>its</strong> enforcement efforts<br />
against AER noncompliance. For example, <strong>FDA</strong> has taken advisory<br />
actions, such as issuing warning letters, against firms that <strong>have</strong> not<br />
reported serious AERs or failed <strong>to</strong> include contact information <strong>to</strong> report an<br />
adverse event on the product label. Second, lawsu<strong>its</strong> <strong>have</strong> publicized the<br />
consequences of adverse events and a firm’s decision not <strong>to</strong> report these<br />
events. Specifically, a number of lawsu<strong>its</strong> <strong>have</strong> been filed against firms<br />
that produced and distributed Hydroxycut—a weight loss supplement<br />
24 GAO-09-250.<br />
Page 16<br />
GAO-13-244 <strong>Dietary</strong> Supplements