Get my PhD Thesis
Get my PhD Thesis
Get my PhD Thesis
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
40 L. Thøgersen, J. Olsen / Chemical Physics Letters 393 (2004) 36–43<br />
0.007<br />
0.006<br />
CCSDT<br />
CCSDTQ<br />
Deviation from FCI energy<br />
0.005<br />
0.004<br />
0.003<br />
0.002<br />
0.001<br />
0<br />
0.9 1 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8<br />
Internuclear distance<br />
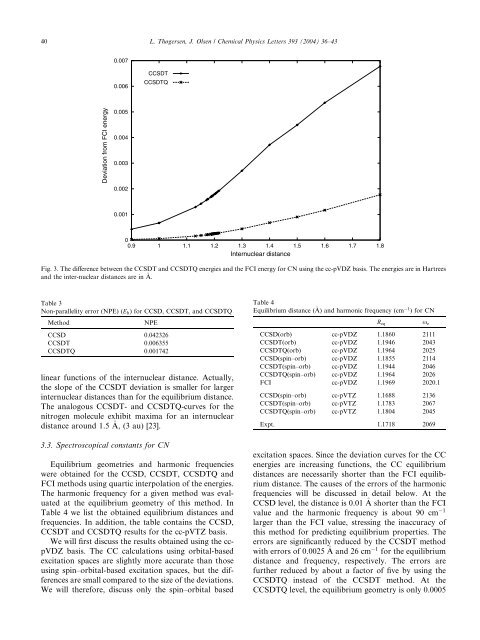
Fig. 3. The difference between the CCSDT and CCSDTQ energies and the FCI energy for CN using the cc-pVDZ basis. The energies are in Hartrees<br />
and the inter-nuclear distances are in A.<br />
Table 3<br />
Non-parallelity error (NPE) (E h ) for CCSD, CCSDT, and CCSDTQ<br />
Method<br />
NPE<br />
CCSD 0.042326<br />
CCSDT 0.006355<br />
CCSDTQ 0.001742<br />
linear functions of the internuclear distance. Actually,<br />
the slope of the CCSDT deviation is smaller for larger<br />
internuclear distances than for the equilibrium distance.<br />
The analogous CCSDT- and CCSDTQ-curves for the<br />
nitrogen molecule exhibit maxima for an internuclear<br />
distance around 1.5 A, (3 au) [23].<br />
3.3. Spectroscopical constants for CN<br />
Equilibrium geometries and harmonic frequencies<br />
were obtained for the CCSD, CCSDT, CCSDTQ and<br />
FCI methods using quartic interpolation of the energies.<br />
The harmonic frequency for a given method was evaluated<br />
at the equilibrium geometry of this method. In<br />
Table 4 we list the obtained equilibrium distances and<br />
frequencies. In addition, the table contains the CCSD,<br />
CCSDT and CCSDTQ results for the cc-pVTZ basis.<br />
We will first discuss the results obtained using the ccpVDZ<br />
basis. The CC calculations using orbital-based<br />
excitation spaces are slightly more accurate than those<br />
using spin–orbital-based excitation spaces, but the differences<br />
are small compared to the size of the deviations.<br />
We will therefore, discuss only the spin–orbital based<br />
Table 4<br />
Equilibrium distance ( A) and harmonic frequency (cm 1 ) for CN<br />
CCSD(orb) cc-pVDZ 1.1860 2111<br />
CCSDT(orb) cc-pVDZ 1.1946 2043<br />
CCSDTQ(orb) cc-pVDZ 1.1964 2025<br />
CCSD(spin–orb) cc-pVDZ 1.1855 2114<br />
CCSDT(spin–orb) cc-pVDZ 1.1944 2046<br />
CCSDTQ(spin–orb) cc-pVDZ 1.1964 2026<br />
FCI cc-pVDZ 1.1969 2020.1<br />
CCSD(spin–orb) cc-pVTZ 1.1688 2136<br />
CCSDT(spin–orb) cc-pVTZ 1.1783 2067<br />
CCSDTQ(spin–orb) cc-pVTZ 1.1804 2045<br />
Expt. 1.1718 2069<br />
excitation spaces. Since the deviation curves for the CC<br />
energies are increasing functions, the CC equilibrium<br />
distances are necessarily shorter than the FCI equilibrium<br />
distance. The causes of the errors of the harmonic<br />
frequencies will be discussed in detail below. At the<br />
CCSD level, the distance is 0.01 A shorter than the FCI<br />
value and the harmonic frequency is about 90 cm 1<br />
larger than the FCI value, stressing the inaccuracy of<br />
this method for predicting equilibrium properties. The<br />
errors are significantly reduced by the CCSDT method<br />
with errors of 0.0025 A and 26 cm 1 for the equilibrium<br />
distance and frequency, respectively. The errors are<br />
further reduced by about a factor of five by using the<br />
CCSDTQ instead of the CCSDT method. At the<br />
CCSDTQ level, the equilibrium geometry is only 0.0005<br />
R eq<br />
x e