Annual General Meeting of the Irish Thoracic Society - IJMS | Irish ...
Annual General Meeting of the Irish Thoracic Society - IJMS | Irish ...
Annual General Meeting of the Irish Thoracic Society - IJMS | Irish ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
41<br />
1<br />
SESSION FOUR ONE<br />
ANNUAL MEETING OF THE IRISH THORACIC SOCIETY • 11 - 12 November 2005 • WESTWOOD HOUSE HOTEL, GALWAY<br />
Session 4: Lung Injury / Inflammation<br />
ANNUAL MEETING OF THE IRISH THORACIC SOCIETY • 11 - 12 November 2005 • WESTWOOD HOUSE HOTEL, GALWAY<br />
SESSION<br />
4SESSION FOUR ONE<br />
4.1<br />
Hypercapnia does not attenuate oxidation in <strong>the</strong> endotoxin<br />
induced acute lung injury<br />
Introduction<br />
Acute Lung Injury (ALI) is a major problem<br />
causing thousands <strong>of</strong> deaths annually worldwide.<br />
Hypercapnic acidosis (HCA) has protective effects in<br />
endotoxin-induced ALI and may have <strong>the</strong>rapeutic<br />
potential. The mechanism <strong>of</strong> this protective action is<br />
unknown; it has been shown in vitro that high CO2<br />
can inhibit peroxynitrite-mediated oxidation, an<br />
important mechanism <strong>of</strong> damage in ALI. We tested<br />
<strong>the</strong> hypo<strong>the</strong>sis that HCA attenuates ALI in vivo by<br />
inhibiting peroxynitrite-mediated oxidation.<br />
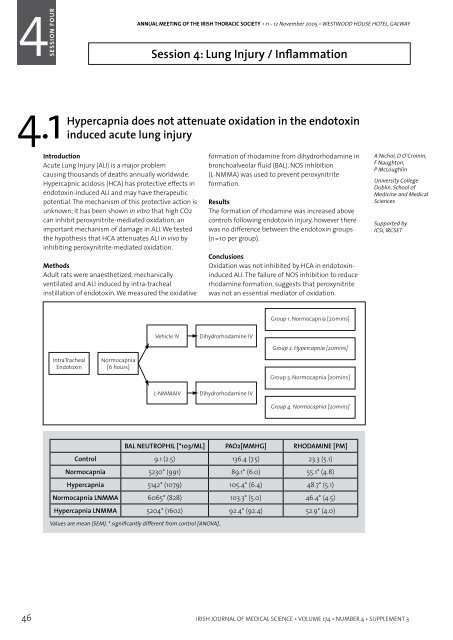
Methods<br />
Adult rats were anaes<strong>the</strong>tized, mechanically<br />
ventilated and ALI induced by intra-tracheal<br />
instillation <strong>of</strong> endotoxin. We measured <strong>the</strong> oxidative<br />
<br />
formation <strong>of</strong> rhodamine from dihydrorhodamine in<br />
bronchoalveolar fluid (BAL). NOS inhibition<br />
(L-NMMA) was used to prevent peroxynitrite<br />
formation.<br />
Results<br />
The formation <strong>of</strong> rhodamine was increased above<br />
controls following endotoxin injury, however <strong>the</strong>re<br />
was no difference between <strong>the</strong> endotoxin groups<br />
(n=10 per group).<br />
Conclusions<br />
Oxidation was not inhibited by HCA in endotoxininduced<br />
ALI. The failure <strong>of</strong> NOS inhibition to reduce<br />
rhodamine formation, suggests that peroxynitrite<br />
was not an essential mediator <strong>of</strong> oxidation.<br />
<br />
<br />
A Nichol, D O’Croinin,<br />
F Naughton,<br />
P McLoughlin<br />
University College<br />
Dublin, School <strong>of</strong><br />
Medicine and Medical<br />
Sciences<br />
Supported by<br />
ICSI, IRCSET<br />
4.2<br />
Up-regulation <strong>of</strong> matrix metalloproteases and ca<strong>the</strong>psins<br />
by neutrophile elastase: a novel hierarchy in protease<br />
regulation<br />
Introduction<br />
Matrix metalloprotease (MMP), ca<strong>the</strong>psin and<br />
neutrophil elastase (NE) activities were measured in<br />
bronchoalveolar lavage (BAL) from individuals with<br />
Alpha-1 Antitrypsin (AAT) deficiency, pneumonia<br />
and healthy controls. We observed correlation<br />
between NE activity and ca<strong>the</strong>psin and MMP activity<br />
postulating that NE might upregulate expression <strong>of</strong><br />
<strong>the</strong>se key proteases and that this upregulation could<br />
be inhibited by treatment with aerosolised plasma<br />
purified AAT (ppAAT).<br />
Methods<br />
Macrophage supernatants were assessed for<br />
ca<strong>the</strong>psin and MMP expression and activity<br />
following stimulation with NE. Ca<strong>the</strong>psin and MMP<br />
activities were assessed in pneumonia and control<br />
BAL and in BAL from AAT deficient patients pre- and<br />
post- aerosolisation <strong>of</strong> ppAAT.<br />
Results<br />
Ca<strong>the</strong>psin and MMP activity are significantly higher<br />
in BAL samples in AAT deficiency and pneumonia<br />
compared to controls and correlate significantly<br />
with NE activity. Treatment with ppAAT significantly<br />
decreases ca<strong>the</strong>psin and MMP activity and<br />
expression in BAL from AAT deficient patients in vivo<br />
and pneumonia patients in vitro.<br />
Conclusions<br />
We describe a novel protease cascade whereby<br />
NE upregulates ca<strong>the</strong>psin and MMP expression/<br />
activation. Neutralization <strong>of</strong> NE by <strong>the</strong> antiprotease,<br />
AAT, inhibits this up-regulation. This<br />
new insight into protease function may facilitate<br />
targeted anti-protease <strong>the</strong>rapy in diseases such as<br />
COPD, CF, pneumonia and A1AT deficiency where NEinduced<br />
ca<strong>the</strong>psin / MMP-mediated inflammation<br />
are a feature.<br />
M Rogan, P Geraghty,<br />
C Greene, M Brantly,<br />
C Taggart, S O’Neill,<br />
NG McElvaney<br />
Pulmonary research<br />
Division, RCSI,<br />
Beaumont Hospital,<br />
Dublin and Dept <strong>of</strong><br />
Medicine, University <strong>of</strong><br />
Florida, USA<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
4.3<br />
Endoplasmic reticulum stress-induced apoptosis and<br />
its inhibition by Tauroursodeoxycholic acid in Alpha-1<br />
antitryspsin deficiency<br />
BAL NEUTROPHIL [*103/ML] PAO2[MMHG] RHODAMINE [PM]<br />
Control 9.1 (2.5) 136.4 (7.5) 23.3 (5.1)<br />
Normocapnia 5230* (991) 89.1* (6.0) 55.1* (4.8)<br />
Hypercapnia 5142* (1079) 105.4* (6.4) 48.7* (5.1)<br />
Normocapnia LNMMA 6065* (828) 103.3* (5.0) 46.4* (4.5)<br />
Hypercapnia LNMMA 5204* (1602) 92.4* (92.4) 52.9* (4.0)<br />
Values are mean (SEM). * significantly different from control [ANOVA]..<br />
<br />
Introduction<br />
Z alpha-1-antitrypsin (AAT) deficiency is a<br />
genetic disease associated with accumulation<br />
<strong>of</strong> misfolded AAT in <strong>the</strong> endoplasmic reticulum<br />
(ER). We investigated both <strong>the</strong> effect <strong>of</strong> ZAAT on<br />
apoptosis, using an in vitro model system <strong>of</strong> ZAAT ER<br />
accumulation, and <strong>the</strong> mechanism <strong>of</strong> inhibition <strong>of</strong><br />
this apoptosis by tauroursodeoxycholic acid (TUDCA).<br />
Method<br />
Apoptosis was induced in HEK293 cells using<br />
thapsigargin, etoposide or by transfection with<br />
a ZAAT cDNA. Cleavage <strong>of</strong> caspases-3,-4 and -7,<br />
cytochrome c release and phosphorylation <strong>of</strong> <strong>the</strong><br />
Bcl-2 family member Bad were assessed by western<br />
immunoblotting. Caspase activities were quantified<br />
by fluorimetry or luminometry, as appropriate.<br />
Apoptosis was demonstrated using TUNEL staining<br />
and cell viability assays were performed. The<br />
inhibitory effects <strong>of</strong> TUDCA were also assessed.<br />
Results<br />
ER accumulation <strong>of</strong> ZAAT, but not normal MAAT,<br />
leads to cleavage and activation <strong>of</strong> caspases -3,-4<br />
and -7. Similar effects were also induced using <strong>the</strong><br />
ER agonist thapsigargin. TUNEL staining <strong>of</strong> ZAATexpressing<br />
cells confirmed <strong>the</strong> presence <strong>of</strong> apoptosis.<br />
Inhibition studies using TUDCA demonstrated its<br />
ability to inhibit caspase-4 and caspase-3/7 activation,<br />
mitochondrial release <strong>of</strong> cytochrome c and apoptosis<br />
induced by ZAAT. TUDCA increased phosphorylation<br />
<strong>of</strong> Bad. Cell viability assays confirmed <strong>the</strong> beneficial<br />
effect <strong>of</strong> TUDCA on cell survival.<br />
Conclusions<br />
Our data shows a new mechanism <strong>of</strong> cell death<br />
in ZAAT deficiency: Activation <strong>of</strong> <strong>the</strong> caspase<br />
cascade and apoptosis via <strong>the</strong> ER-specific caspase-<br />
4. Inhibition studies using TUDCA demonstrate<br />
its ability to inhibit caspase activation via <strong>the</strong><br />
phosphorylation <strong>of</strong> Bad and implicate it as a<br />
potential <strong>the</strong>rapeutic agent in AAT deficiency.<br />
S Miller, C Greene,<br />
C Taggart, C McElvaney,<br />
S O’Neill<br />
Dept <strong>of</strong> Medicine,<br />
Respiratory Research<br />
Division, RCSI<br />
Education and<br />
Research Centre,<br />
Beaumont Hospital,<br />
Dublin<br />
46 IRISH JOURNAL OF MEDICAL SCIENCE • VOLUME 174 • NUMBER 4 • SUPPLEMENT 3<br />
IRISH JOURNAL OF MEDICAL SCIENCE • VOLUME 174 • NUMBER 4 • SUPPLEMENT 3 47