02-a Wet etching - Caltech Micromachining Laboratory
02-a Wet etching - Caltech Micromachining Laboratory
02-a Wet etching - Caltech Micromachining Laboratory
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
"...-~-: 1804' .' -J. Electrochem. SoC.: SOLID-STATE SCIENCE AND TECHNOLOGY December<br />
,".:: , :." '- --.<br />
~_£~~~~, : ~ -.The <strong>etching</strong> was done in a Teflon beaker 1 in. in -: : ~<br />
."-:'~;'-.:.- -diameter and 2112 in. deep. Agitation was provided by<br />
~ ::.' an electric stirrer equipped with a polyethylene paddle. ;j<br />
,;;-=.~'~ The dice were etched one at a time in 10 cm3 of :! .6<br />
..,1:':~: '. solution, and duplicate results were obtained by re- ~<br />
~;:-~~' ,~'- peating the en~ire experiment. To quench the reaction ItJ<br />
-:.<br />
1:;'.:<br />
at the proper tIme, a large volume of water was poured<br />
into the beaker. The <strong>etching</strong> technique was later mod-<br />
~ 12<br />
:I:<br />
'::','!':;<br />
.;~.<br />
ified when the ~igher concentration reagent~ we:e<br />
used. Here the dIce were etched two at a tIme, In ~<br />
"j':. -20 cm3 of solution, while encased in a small perforated ~<br />
". ':. polyethylene basket. The basket and dice were agitated ~<br />
for the required time in the solution, then immersed z:<br />
in a beaker of cold water to quench the reaction. The I-<br />
agitating action consisted of approximately 150 vertical<br />
strokes per minute, with the <strong>etching</strong> jig rotated<br />
". betv.:een th~ thumb and middle finger as rapidly as 15 20 25<br />
possIble d~Ing the up and do":",n strokes.<br />
TIME (SECONDS)<br />
All etchIng was performed In a constant temperature<br />
(A)<br />
bath regulated at 25.00. =- 0.<strong>02</strong>.C. Unfortunately,<br />
.: .the heat transmission of polyethylene and Teflon is<br />
.~:<br />
i:';:<br />
so poor that the temperature of the bath could not be<br />
realized in the .etch solution, and even after long<br />
Vi<br />
=<br />
:';.:;: .periods of equilibration the maximum initial e~h ~ 12<br />
:::; ~J temperature was only 24.70.C. .AS; a further Ca:n-<br />
..,.;; ~ lequence of the poor heat transInlSSlOn of the <strong>etching</strong><br />
~<br />
z<br />
":":<br />
~.~~<br />
container, the more rapid etches were run under<br />
essentially adiabatic conditions.<br />
~<br />
(.) 8<br />
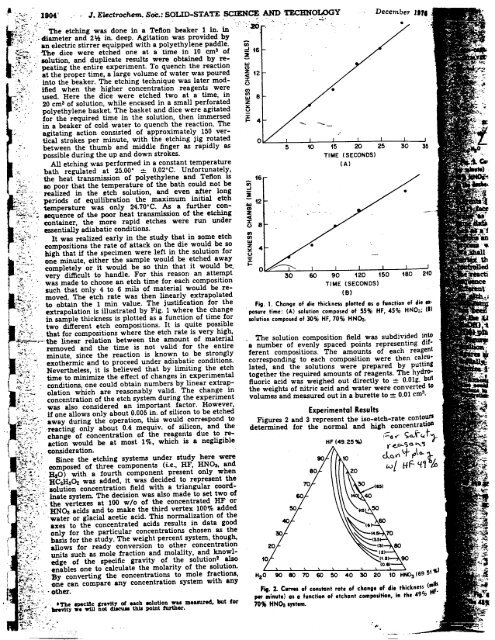
~~;;,::: It was realized early in the study that in some etch ~<br />
..:0,: .,<br />
':::',<br />
compositions the rate of attack on the die would be so<br />
high t~at if t~e specimen were left in the solution for ~ 4<br />
~:,.,,;.,~. one mlnute, eIther the sample would be etched away x:<br />
'.~:,,::.-i:Ompletely or it would be so thin that it would be. I-<br />
,:_.:' :j:' '. was very made difficult to choose to handle. etch For time this for reason each composition an attempt<br />
90 120 150<br />
:",-:..such that only 4 to 6 mils of material would be re- TIME (SECONDS)<br />
,4:::' moved. The etch rate was then linearly extrapolated (8)<br />
of "', ,' c L - loft d f t. f d .- II .<br />
:~;;.~';<br />
'.:, to tobtain 1 t... the 1 11 min t value. ted b The Fi justification 1 h th for h the F... 1 C hang - ~ rn ~n~.s peas a unc Ion 0 ,.<br />
~-.,: -:~.:, ..', .ex rapo a IO~ IS 1 us. ra y g. w ~re e.c ange posure time: (A) solution composed of 55% HF. 45% HNO3; (I)<br />
::". .In two sam.ple different thickness etch 15 composItIons. plotte;~ as a It fu~ctIon.of 15 qwte tlme.for posslble solution composed of 30% HF . 70% HH03.<br />
~:~-' .that for compositions where the etch rate is very high,<br />
~~,--'.the linear relation between the amount of material -The solution composition field was subdivided into<br />
-:,~:" removed and the time is not valid for the entire a number of evenly spaced points representing dif-<br />
.f ."..:.- minute, since the reaction is known to be strongly ferent compositions. The amounts of each reagent<br />
.~:j.:~:,: ~c exothermic and to proceed under adiabatic conditions. corresponding to each composition were then calc:-u-<br />
~';~,'. Nevertheless, it is believed that by limiting the etch lated, and the solutions were prepared by puttIng<br />
;~J,~;~ ~. time to minimize the effect of changes in experimental together the required amounts of reagents. The hydro-<br />
7;.:.:;; -: conditions, one could obtain numbers by linear extrap- fluoric acid was weighed out directly to =- O.Olg. but<br />
i",:~~ olation which are reasonably valid. The change in the weights of nitric acid and water were converted to<br />
~:~' ".: concentration of the etch system during the experiment volumes and measured out in a burette to: 0.01 cm:J.<br />
,~:..'{:~:.- was also considered an important factor. However,<br />
-.~.;;:.. if one allows only about 0.005 in. of silicon to be etched<br />
Experimental Results<br />
~'::t'~':~' --away. during the operation, t~ would. .correspond to Figures 2 and 3 represent the iso-etch-rate contours<br />
:",:~.'~:";.: reactIng only about ~.4 mequlV. of SIlIcon, and the determined for the normal and high concentratioD<br />
'~;.'~;:'~' ;"";:-;c' '; change action would of concentration be most of 10;0, the which reagents is a due negligible to re- !-o