Primer on Automobile Fuel Efficiency and Emissions - Pollution Probe
Primer on Automobile Fuel Efficiency and Emissions - Pollution Probe
Primer on Automobile Fuel Efficiency and Emissions - Pollution Probe
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
h<br />
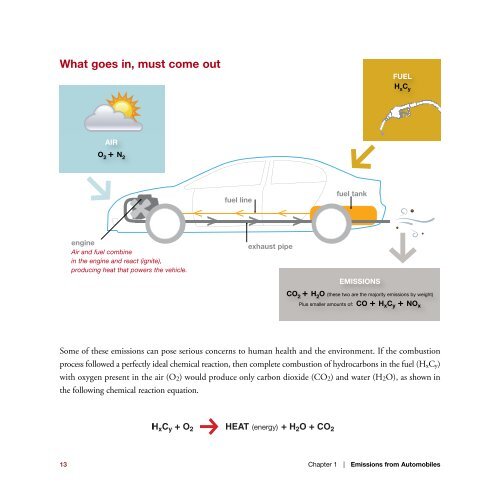
What goes in, must come out<br />
FUEL<br />
H x C y<br />
AIR<br />
O 2 + N<br />
l<br />
2<br />
fuel line<br />
c<br />
fuel tank<br />
engine<br />
Air <strong>and</strong> fuel combine<br />
in the engine <strong>and</strong> react (ignite),<br />
producing heat that powers the vehicle.<br />
exhaust pipe<br />
EMISSIONS<br />
CO 2 + H 2 O (these two are the majority emissi<strong>on</strong>s by weight)<br />
Plus smaller amounts of: CO + H x C y + NO x<br />
Some of these emissi<strong>on</strong>s can pose serious c<strong>on</strong>cerns to human health <strong>and</strong> the envir<strong>on</strong>ment. If the combusti<strong>on</strong><br />
process followed a perfectly ideal chemical reacti<strong>on</strong>, then complete combusti<strong>on</strong> of hydrocarb<strong>on</strong>s in the fuel (H x Cy)<br />
with oxygen present in the air (O 2 ) would produce <strong>on</strong>ly carb<strong>on</strong> dioxide (CO2) <strong>and</strong> water (H2O), as shown in<br />
the following chemical reacti<strong>on</strong> equati<strong>on</strong>.<br />
13<br />
Chapter 1 | Emissi<strong>on</strong>s from <strong>Automobile</strong>s