Erik Lindahl
Erik Lindahl
Erik Lindahl
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
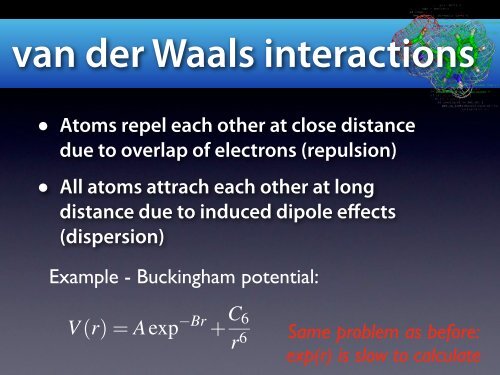
van der Waals interactions<br />
• Atoms repel each other at close distance<br />
due to overlap of electrons (repulsion)<br />
• All atoms attrach each other at long<br />
distance due to induced dipole effects<br />
(dispersion)<br />
Example - Buckingham potential:<br />
V (r)=Aexp Br + C 6<br />
r 6<br />
Same problem as before:<br />
exp(r) is slow to calculate
















![[VAR]=Notes on variational calculus](https://img.yumpu.com/35639168/1/190x245/varnotes-on-variational-calculus.jpg?quality=85)