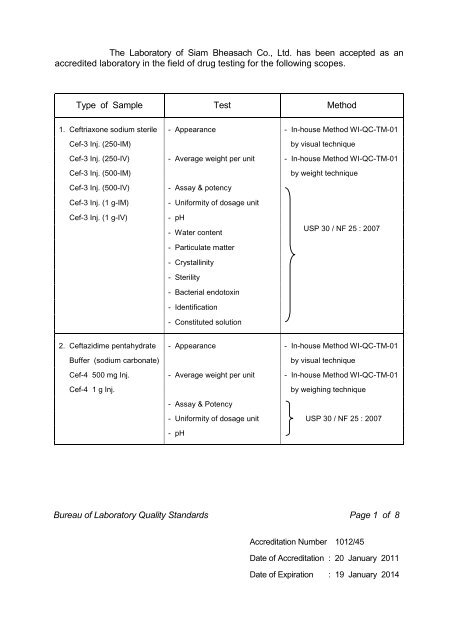
The Laboratory of Siam Bheasach Co., Ltd. has been accepted as ...
The Laboratory of Siam Bheasach Co., Ltd. has been accepted as ...
The Laboratory of Siam Bheasach Co., Ltd. has been accepted as ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>The</strong> <strong>Laboratory</strong> <strong>of</strong> <strong>Siam</strong> <strong>Bhe<strong>as</strong>ach</strong> <strong>Co</strong>., <strong>Ltd</strong>. <strong>h<strong>as</strong></strong> <strong>been</strong> <strong>accepted</strong> <strong>as</strong> an<br />
accredited laboratory in the field <strong>of</strong> drug testing for the following scopes.<br />
Type <strong>of</strong> Sample Test Method<br />
1. Ceftriaxone sodium sterile - Appearance - In-house Method WI-QC-TM-01<br />
Cef-3 Inj. (250-IM)<br />
by visual technique<br />
Cef-3 Inj. (250-IV) - Average weight per unit - In-house Method WI-QC-TM-01<br />
Cef-3 Inj. (500-IM)<br />
by weight technique<br />
Cef-3 Inj. (500-IV)<br />
- Assay & potency<br />
Cef-3 Inj. (1 g-IM)<br />
Cef-3 Inj. (1 g-IV)<br />
- Uniformity <strong>of</strong> dosage unit<br />
- pH<br />
- Water content<br />
- Particulate matter<br />
- Crystallinity<br />
- Sterility<br />
- Bacterial endotoxin<br />
- Identification<br />
- <strong>Co</strong>nstituted solution<br />
USP 30 / NF 25 : 2007<br />
2. Ceftazidime pentahydrate - Appearance - In-house Method WI-QC-TM-01<br />
Buffer (sodium carbonate)<br />
by visual technique<br />
Cef-4 500 mg Inj. - Average weight per unit - In-house Method WI-QC-TM-01<br />
Cef-4 1 g Inj.<br />
by weighing technique<br />
- Assay & Potency<br />
- Uniformity <strong>of</strong> dosage unit USP 30 / NF 25 : 2007<br />
- pH<br />
Bureau <strong>of</strong> <strong>Laboratory</strong> Quality Standards Page 1 <strong>of</strong> 8<br />
Accreditation Number 1012/45<br />
Date <strong>of</strong> Accreditation : 20 January 2011<br />
Date <strong>of</strong> Expiration : 19 January 2014
<strong>The</strong> <strong>Laboratory</strong> <strong>of</strong> <strong>Siam</strong> <strong>Bhe<strong>as</strong>ach</strong> <strong>Co</strong>., <strong>Ltd</strong>. <strong>h<strong>as</strong></strong> <strong>been</strong> <strong>accepted</strong> <strong>as</strong> an<br />
accredited laboratory in the field <strong>of</strong> drug testing for the following scopes.<br />
Type <strong>of</strong> Sample Test Method<br />
2. Ceftazidime pentahydrate - Loss on drying<br />
Buffer (sodium carbonate)<br />
Cef-4 500 mg Inj.<br />
Cef-4 1 g Inj.<br />
(<strong>Co</strong>ntinued)<br />
- Particulate matter<br />
- Pyridine content<br />
- Sterility<br />
- Bacterial endotoxin<br />
- <strong>Co</strong>nstituted solution<br />
- Identification<br />
- Sodium carbonate content<br />
USP 30 / NF 25 : 2007<br />
3. Cefotaxime sodium sterile - Appearance - In-house Method WI-QC-TM-01<br />
Claraxim 0.5 gm Inj.<br />
by visual technique<br />
Claraxim 1.0 gm Inj. - Average weight per unit - In-house Method WI-QC-TM-01<br />
by weight technique<br />
- Assay & Potency<br />
- Uniformity <strong>of</strong> dosage unit<br />
- pH<br />
- Loss on drying<br />
USP 30 / NF 25 : 2007<br />
- Particulate matter<br />
- Sterility<br />
- Bacterial endotoxin<br />
- <strong>Co</strong>nstituted solution<br />
- Identification<br />
- Chromatographic purity<br />
Bureau <strong>of</strong> <strong>Laboratory</strong> Quality Standards Page 2 <strong>of</strong> 8<br />
Accreditation Number 1012/45<br />
Date <strong>of</strong> Accreditation : 20 January 2011<br />
Date <strong>of</strong> Expiration : 19 January 2014
<strong>The</strong> <strong>Laboratory</strong> <strong>of</strong> <strong>Siam</strong> <strong>Bhe<strong>as</strong>ach</strong> <strong>Co</strong>., <strong>Ltd</strong>. <strong>h<strong>as</strong></strong> <strong>been</strong> <strong>accepted</strong> <strong>as</strong> an<br />
accredited laboratory in the field <strong>of</strong> drug testing for the following scopes.<br />
Type <strong>of</strong> Sample Test Method<br />
4. Acyclovir - Average weight per unit - In-house Method WI-QC-TM-01<br />
Vilerm 200 mg Tablet<br />
Vilerm 400 mg Tablet<br />
Vilerm 800 mg Tablet<br />
- Uniformity <strong>of</strong> dosage unit<br />
(Weight variation)<br />
- Assay<br />
- Friability<br />
- Disintegration<br />
- Dissolution<br />
- Related compounds<br />
* Guanine<br />
* Any other impurity<br />
- Identification<br />
by weight technique<br />
USP 32 / NF 27 : 2009<br />
5. Enalapril maleate - Average weight per unit - In-house Method WI-QC-TM-01<br />
Nalopril 5 mg Tablet by weighing technique<br />
Nalopril 10 mg Tablet<br />
Nalopril 20 mg Tablet<br />
- Uniformity <strong>of</strong> dosage unit<br />
(<strong>Co</strong>ntent Uniformity)<br />
- Assay<br />
- Disintegration<br />
USP 33 / NF 28 : 2010<br />
- Related compounds (Total)<br />
- Dissolution<br />
- Identification<br />
- Friability<br />
Bureau <strong>of</strong> <strong>Laboratory</strong> Quality Standards Page 3 <strong>of</strong> 8<br />
Accreditation Number 1012/45<br />
Date <strong>of</strong> Accreditation : 20 January 2011<br />
Date <strong>of</strong> Expiration : 19 January 2014
<strong>The</strong> <strong>Laboratory</strong> <strong>of</strong> <strong>Siam</strong> <strong>Bhe<strong>as</strong>ach</strong> <strong>Co</strong>., <strong>Ltd</strong>. <strong>h<strong>as</strong></strong> <strong>been</strong> <strong>accepted</strong> <strong>as</strong> an<br />
accredited laboratory in the field <strong>of</strong> drug testing for the following scopes.<br />
Type <strong>of</strong> Sample Test Method<br />
6. Sulcef Injection - Appearance - In-house Method WI-QC-TM-01<br />
Cefoperazone Sodium<br />
by visual technique<br />
Sulbactam Sodium - Average weight per unit - In-house Method WI-QC-TM-01<br />
by weighing technique<br />
- Uniformity <strong>of</strong> dosage unit<br />
(<strong>Co</strong>ntent Uniformity)<br />
- pH (25% solution)<br />
- Water content<br />
- <strong>Co</strong>nstituted solution USP 31 / NF 26 : 2008<br />
- Particulate matter<br />
- Sterility<br />
- Bacterial endotoxin<br />
- Identification<br />
- Chromatographic purity - In-house Method WI-QC-<br />
- Assay and Potency TM-01 by HPLC technique<br />
7. Isosorbide dinitrate - Average weight per unit - In-house Method WI-QC-TM-01<br />
Hartsorb 5 mg Tablet by weighing technique<br />
(Sublingual)<br />
Hartsorb 10 mg Tablet<br />
Hartsorb 30 mg Tablet<br />
Bureau <strong>of</strong> <strong>Laboratory</strong> Quality Standards Page 4 <strong>of</strong> 8<br />
Accreditation Number 1012/45<br />
Date <strong>of</strong> Accreditation : 20 January 2011<br />
Date <strong>of</strong> Expiration : 19 January 2014
<strong>The</strong> <strong>Laboratory</strong> <strong>of</strong> <strong>Siam</strong> <strong>Bhe<strong>as</strong>ach</strong> <strong>Co</strong>., <strong>Ltd</strong>. <strong>h<strong>as</strong></strong> <strong>been</strong> <strong>accepted</strong> <strong>as</strong> an<br />
accredited laboratory in the field <strong>of</strong> drug testing for the following scopes.<br />
Type <strong>of</strong> Sample Test Method<br />
7. Isosorbide dinitrate - Assay<br />
Hartsorb 5 mg Tablet - Uniformity <strong>of</strong> dosage unit<br />
(Sublingual)<br />
(<strong>Co</strong>ntent Uniformity)<br />
USP 33 / NF 28 : 2010<br />
Hartsorb 10 mg Tablet<br />
Hartsorb 30 mg Tablet<br />
(<strong>Co</strong>ntinued)<br />
- Friability<br />
- Disintegration<br />
- Dissolution<br />
- Identification<br />
8. Simv<strong>as</strong>tatin - Average weight per unit - In-house Method WI-QC-TM-01<br />
Lochol 10 mg Tablet<br />
Lochol 20 mg Tablet<br />
Lochol 40 mg Tablet<br />
Lochol 80 mg Tablet<br />
- Assay<br />
- Uniformity <strong>of</strong> dosage unit<br />
(<strong>Co</strong>ntent Uniformity)<br />
- Disintegration<br />
- Dissolution<br />
- Identification<br />
by weight technique<br />
USP 31 / NF 26 : 2008<br />
9. Sterile water for injection - Chloride<br />
- Sulfate<br />
- Calcium USP 30 / NF 25 : 2007<br />
- Carbondioxide<br />
- Oxidizable substance<br />
Bureau <strong>of</strong> <strong>Laboratory</strong> Quality Standards Page 5 <strong>of</strong> 8<br />
Accreditation Number 1012/45<br />
Date <strong>of</strong> Accreditation : 20 January 2011<br />
Date <strong>of</strong> Expiration : 19 January 2014
<strong>The</strong> <strong>Laboratory</strong> <strong>of</strong> <strong>Siam</strong> <strong>Bhe<strong>as</strong>ach</strong> <strong>Co</strong>., <strong>Ltd</strong>. <strong>h<strong>as</strong></strong> <strong>been</strong> <strong>accepted</strong> <strong>as</strong> an<br />
accredited laboratory in the field <strong>of</strong> drug testing for the following scopes.<br />
Type <strong>of</strong> Sample Test Method<br />
9. Sterile water for injection - pH<br />
(<strong>Co</strong>ntinued)<br />
- Volume in container<br />
- Particulate matter<br />
- Sterility USP 30 / NF 25 : 2007<br />
- Bacterial endotoxin<br />
- Ammonia<br />
10. Meropenem injection - Assay Meropenem<br />
(Mapenem 0.5 g injection,<br />
Mapenem 1.0 g injection)<br />
- Uniformity <strong>of</strong> dosage unit<br />
- pH<br />
- Loss on drying<br />
- Particulate matter<br />
USP 32 / NF 27 : 2009<br />
- <strong>Co</strong>ntent <strong>of</strong> sodium<br />
- Chromatographic purity<br />
- <strong>Co</strong>nstituted solution<br />
- Sterility test<br />
- Bacterial endotoxin<br />
- Identification<br />
Bureau <strong>of</strong> <strong>Laboratory</strong> Quality Standards Page 6 <strong>of</strong> 8<br />
Accreditation Number 1012/45<br />
Date <strong>of</strong> Accreditation : 20 January 2011<br />
Date <strong>of</strong> Expiration : 19 January 2014
<strong>The</strong> <strong>Laboratory</strong> <strong>of</strong> <strong>Siam</strong> <strong>Bhe<strong>as</strong>ach</strong> <strong>Co</strong>., <strong>Ltd</strong>. <strong>h<strong>as</strong></strong> <strong>been</strong> <strong>accepted</strong> <strong>as</strong> an<br />
accredited laboratory in the field <strong>of</strong> drug testing for the following scopes.<br />
Type <strong>of</strong> Sample Test Method<br />
11. Powder injection<br />
- Cavumox 1.2 g<br />
(Amoxicillin sodium +<br />
Clavulanic acid)<br />
- Cavumox 0.6 g<br />
(Amoxicillin sodium +<br />
Clavulanic acid)<br />
- Fazolin<br />
(Cefazolin sodium)<br />
- Cefxitin<br />
(Cefoxitin sodium)<br />
Sterility test<br />
Bacterial endotoxin test<br />
USP 32 / NF 27 : 2009<br />
- Furoxime<br />
(Cefuroxime sodium)<br />
- Sulam<br />
(Cefoperazone sodium +<br />
Sulbactam sodium)<br />
- Vilerm<br />
(Acyclovir sodium)<br />
- O – sid<br />
(Omeprazole sodium)<br />
Bureau <strong>of</strong> <strong>Laboratory</strong> Quality Standards Page 7 <strong>of</strong> 8<br />
Accreditation Number 1012/45<br />
Date <strong>of</strong> Accreditation : 20 January 2011<br />
Date <strong>of</strong> Expiration : 19 January 2014
<strong>The</strong> <strong>Laboratory</strong> <strong>of</strong> <strong>Siam</strong> <strong>Bhe<strong>as</strong>ach</strong> <strong>Co</strong>., <strong>Ltd</strong>. <strong>h<strong>as</strong></strong> <strong>been</strong> <strong>accepted</strong> <strong>as</strong> an<br />
accredited laboratory in the field <strong>of</strong> drug testing for the following scopes.<br />
Type <strong>of</strong> Sample Test Method<br />
12. Small volume injection<br />
- Dosanac<br />
(Dicl<strong>of</strong>enac sodium)<br />
- Furetic – S<br />
(Furosemide sodium),<br />
- Inopin (Dopamine HCl),<br />
- Nausil (Metoclopramide HCl), Sterility test USP 32 / NF 27 : 2009<br />
- Onsia (Ondansetron HCl), Bacterial endotoxin test<br />
- Zantidon (Ranitidine HCl),<br />
- Lidocaine HCl 1%<br />
(Lidocaine HCl),<br />
- Solvent for O – Sid<br />
(Polyethylene Glycol 400),<br />
- Flucozole (Fluconazole)<br />
13. Large volume injection<br />
- Metrolex (Metronidazole), Sterility test USP 32 / NF 27 : 2009<br />
- Glyceol (Glycerine) Bacterial endotoxin test<br />
Bureau <strong>of</strong> <strong>Laboratory</strong> Quality Standards Page 8 <strong>of</strong> 8<br />
Accreditation Number 1012/45<br />
Date <strong>of</strong> Accreditation : 20 January 2011<br />
Date <strong>of</strong> Expiration : 19 January 2014