Combinatorial and High-Throughput Screening of Materials ...
Combinatorial and High-Throughput Screening of Materials ...
Combinatorial and High-Throughput Screening of Materials ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
ACS <strong>Combinatorial</strong> Science<br />
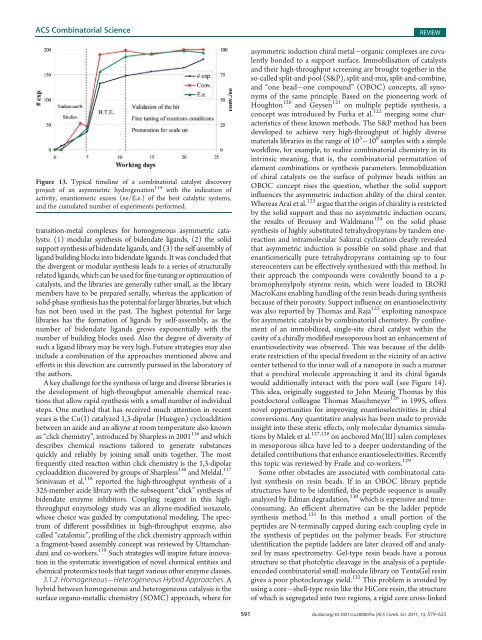
Figure 13. Typical timeline <strong>of</strong> a combinatorial catalyst discovery<br />
project <strong>of</strong> an asymmetric hydrogenation 114 with the indication <strong>of</strong><br />
activity, enantiomeric excess (ee/E.e.) <strong>of</strong> the best catalytic systems,<br />
<strong>and</strong> the cumulated number <strong>of</strong> experiments performed.<br />
transition-metal complexes for homogeneous asymmetric catalysts:<br />
(1) modular synthesis <strong>of</strong> bidendate lig<strong>and</strong>s, (2) the solid<br />
support synthesis <strong>of</strong> bidendate lig<strong>and</strong>s, <strong>and</strong> (3) the self-assembly <strong>of</strong><br />
lig<strong>and</strong> building blocks into bidendate lig<strong>and</strong>s. It was concluded that<br />
the divergent or modular synthesis leads to a series <strong>of</strong> structurally<br />
related lig<strong>and</strong>s, which can be used for fine-tuning or optimization <strong>of</strong><br />
catalysts, <strong>and</strong> the libraries are generally rather small, as the library<br />
members have to be prepared serially, whereas the application <strong>of</strong><br />
solid-phase synthesis has the potential for larger libraries, but which<br />
has not been used in the past. The highest potential for large<br />
libraries has the formation <strong>of</strong> lig<strong>and</strong>s by self-assembly, as the<br />
number <strong>of</strong> bidendate lig<strong>and</strong>s grows exponentially with the<br />
number <strong>of</strong> building blocks used. Also the degree <strong>of</strong> diversity <strong>of</strong><br />
such a lig<strong>and</strong> library may be very high. Future strategies may also<br />
include a combination <strong>of</strong> the approaches mentioned above <strong>and</strong><br />
efforts in this direction are currently pursued in the laboratory <strong>of</strong><br />
the authors.<br />
A key challenge for the synthesis <strong>of</strong> large <strong>and</strong> diverse libraries is<br />
the development <strong>of</strong> high-throughput amenable chemical reactions<br />
that allow rapid synthesis with a small number <strong>of</strong> individual<br />
steps. One method that has received much attention in recent<br />
years is the Cu(I) catalyzed 1,3-dipolar (Huisgen) cycloaddition<br />
between an azide <strong>and</strong> an alkyne at room temperature also known<br />
as “click chemistry”, introduced by Sharpless in 2001 116 <strong>and</strong> which<br />
describes chemical reactions tailored to generate substances<br />
quickly <strong>and</strong> reliably by joining small units together. The most<br />
frequently cited reaction within click chemistry is the 1,3-dipolar<br />
cycloaddition discovered by groups <strong>of</strong> Sharpless 116 <strong>and</strong> Meldal. 117<br />
Srinivasan et al. 118 reported the high-throughput synthesis <strong>of</strong> a<br />
325-member azide library with the subsequent “click” synthesis <strong>of</strong><br />
bidendate enzyme inhibitors. Coupling reagent in this highthroughput<br />
enzymology study was an alkyne-modified isoxazole,<br />
whose choice was guided by computational modeling. The spectrum<br />
<strong>of</strong> different possibilities in high-throughput enzyme, also<br />
called “catalomic”,pr<strong>of</strong>iling <strong>of</strong> the click chemistry approach within<br />
a fragment-based assembly concept was reviewed by Uttamch<strong>and</strong>ani<br />
<strong>and</strong> co-workers. 119 Such strategies will inspire future innovation<br />
in the systematic investigation <strong>of</strong> novel chemical entities <strong>and</strong><br />
chemical proteomics tools that target various other enzyme classes.<br />
3.1.2. Homogeneous Heterogeneous Hybrid Approaches. A<br />
hybrid between homogeneous <strong>and</strong> heterogeneous catalysis is the<br />
surface organo-metallic chemistry (SOMC) approach, where for<br />
REVIEW<br />
asymmetric induction chiral metal organic complexes are covalently<br />
bonded to a support surface. Immobilisation <strong>of</strong> catalysts<br />
<strong>and</strong> their high-throughput screening are brought together in the<br />
so-called split-<strong>and</strong>-pool (S&P), split-<strong>and</strong>-mix, split-<strong>and</strong>-combine,<br />
<strong>and</strong> “one bead one compound” (OBOC) concepts, all synonyms<br />
<strong>of</strong> the same principle. Based on the pioneering work <strong>of</strong><br />
Houghton 120 <strong>and</strong> Geysen 121 on multiple peptide synthesis, a<br />
concept was introduced by Furka et al. 122 merging some characteristics<br />
<strong>of</strong> these known methods. The S&P method has been<br />
developed to achieve very high-throughput <strong>of</strong> highly diverse<br />
materials libraries in the range <strong>of</strong> 10 3 10 8 samples with a simple<br />
workflow, for example, to realize combinatorial chemistry in its<br />
intrinsic meaning, that is, the combinatorial permutation <strong>of</strong><br />
element combinations or synthesis parameters. Immobilization<br />
<strong>of</strong> chiral catalysts on the surface <strong>of</strong> polymer beads within an<br />
OBOC concept rises the question, whether the solid support<br />
influences the asymmetric induction ability <strong>of</strong> the chiral center.<br />
Whereas Arai et al. 123 argue that the origin <strong>of</strong> chirality is restricted<br />
by the solid support <strong>and</strong> thus no asymmetric induction occurs,<br />
the results <strong>of</strong> Broussy <strong>and</strong> Waldmann 124 on the solid phase<br />
synthesis <strong>of</strong> highly substituted tetrahydropyrans by t<strong>and</strong>em enereaction<br />
<strong>and</strong> intramolecular Sakurai cyclization clearly revealed<br />
that asymmetric induction is possible on solid phase <strong>and</strong> that<br />
enantiomerically pure tetrahydropyrans containing up to four<br />
stereocenters can be effectively synthesized with this method. In<br />
their approach the compounds were covalently bound to a p-<br />
bromophenylpoly styrene resin, which were loaded in IRORI<br />
MacroKans enabling h<strong>and</strong>ling <strong>of</strong> the resin beads during synthesis<br />
because <strong>of</strong> their porosity. Support influence on enantioselectivity<br />
was also reported by Thomas <strong>and</strong> Raja 125 exploiting nanospace<br />
for asymmetric catalysis by combinatorial chemistry. By confinement<br />
<strong>of</strong> an immobilized, single-site chiral catalyst within the<br />
cavity <strong>of</strong> a chirally modified mesoporous host an enhancement <strong>of</strong><br />
enantioselectivity was observed. This was because <strong>of</strong> the deliberate<br />
restriction <strong>of</strong> the special freedom in the vicinity <strong>of</strong> an active<br />
center tethered to the inner wall <strong>of</strong> a nanopore in such a manner<br />
that a prochiral molecule approaching it <strong>and</strong> its chiral lig<strong>and</strong>s<br />
would additionally interact with the pore wall (see Figure 14).<br />
This idea, originally suggested to John Meurig Thomas by this<br />
postdoctoral colleague Thomas Maschmeyer 126 in 1995, <strong>of</strong>fers<br />
novel opportunities for improving enantioselectivities in chiral<br />
conversions. Any quantitative analysis has been made to provide<br />
insight into these steric effects, only molecular dynamics simulations<br />
by Malek et al. 127,128 on anchored Mn(III) salen complexes<br />
in mesoporous silica have led to a deeper underst<strong>and</strong>ing <strong>of</strong> the<br />
detailed contributions that enhance enantioselectivities. Recently<br />
this topic was reviewed by Fraile <strong>and</strong> co-workers. 129<br />
Some other obstacles are associated with combinatorial catalyst<br />
synthesis on resin beads. If in an OBOC library peptide<br />
structures have to be identified, the peptide sequence is usually<br />
analyzed by Edman degradation, 130 which is expensive <strong>and</strong> timeconsuming.<br />
An efficient alternative can be the ladder peptide<br />
synthesis method. 131 In this method a small portion <strong>of</strong> the<br />
peptides are N-terminally capped during each coupling cycle in<br />
the synthesis <strong>of</strong> peptides on the polymer beads. For structure<br />
identification the peptide ladders are later cleaved <strong>of</strong>f <strong>and</strong> analyzed<br />
by mass spectrometry. Gel-type resin beads have a porous<br />
structure so that photolytic cleavage in the analysis <strong>of</strong> a peptideencoded<br />
combinatorial small molecule library on TentaGel resin<br />
gives a poor photocleavage yield. 132 This problem is avoided by<br />
using a core shell-type resin like the HiCore resin, the structure<br />
<strong>of</strong> which is segregated into two regions, a rigid core cross-linked<br />
591 dx.doi.org/10.1021/co200007w |ACS Comb. Sci. 2011, 13, 579–633