Combinatorial and High-Throughput Screening of Materials ...
Combinatorial and High-Throughput Screening of Materials ...
Combinatorial and High-Throughput Screening of Materials ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
ACS <strong>Combinatorial</strong> Science<br />
REVIEW<br />
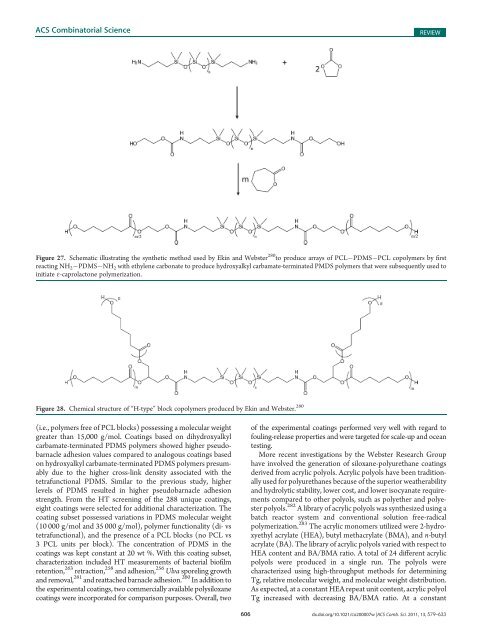
Figure 27. Schematic illustrating the synthetic method used by Ekin <strong>and</strong> Webster 280 to produce arrays <strong>of</strong> PCL PDMS PCL copolymers by first<br />
reacting NH 2 PDMS NH 2 with ethylene carbonate to produce hydroxyalkyl carbamate-terminated PMDS polymers that were subsequently used to<br />
initiate ε-caprolactone polymerization.<br />
Figure 28. Chemical structure <strong>of</strong> “H-type” block copolymers produced by Ekin <strong>and</strong> Webster. 280<br />
(i.e., polymers free <strong>of</strong> PCL blocks) possessing a molecular weight<br />
greater than 15,000 g/mol. Coatings based on dihydroxyalkyl<br />
carbamate-terminated PDMS polymers showed higher pseudobarnacle<br />
adhesion values compared to analogous coatings based<br />
on hydroxyalkyl carbamate-terminated PDMS polymers presumably<br />
due to the higher cross-link density associated with the<br />
tetrafunctional PDMS. Similar to the previous study, higher<br />
levels <strong>of</strong> PDMS resulted in higher pseudobarnacle adhesion<br />
strength. From the HT screening <strong>of</strong> the 288 unique coatings,<br />
eight coatings were selected for additional characterization. The<br />
coating subset possessed variations in PDMS molecular weight<br />
(10 000 g/mol <strong>and</strong> 35 000 g/mol), polymer functionality (di- vs<br />
tetrafunctional), <strong>and</strong> the presence <strong>of</strong> a PCL blocks (no PCL vs<br />
3 PCL units per block). The concentration <strong>of</strong> PDMS in the<br />
coatings was kept constant at 20 wt %. With this coating subset,<br />
characterization included HT measurements <strong>of</strong> bacterial bi<strong>of</strong>ilm<br />
retention, 263 retraction, 258 <strong>and</strong> adhesion, 256 Ulva sporeling growth<br />
<strong>and</strong> removal, 281 <strong>and</strong> reattached barnacle adhesion. 260 In addition to<br />
the experimental coatings, two commercially available polysiloxane<br />
coatings were incorporated for comparison purposes. Overall, two<br />
<strong>of</strong> the experimental coatings performed very well with regard to<br />
fouling-release properties <strong>and</strong> were targeted for scale-up <strong>and</strong> ocean<br />
testing.<br />
More recent investigations by the Webster Research Group<br />
have involved the generation <strong>of</strong> siloxane-polyurethane coatings<br />
derived from acrylic polyols. Acrylic polyols have been traditionally<br />
used for polyurethanes because <strong>of</strong> the superior weatherability<br />
<strong>and</strong> hydrolytic stability, lower cost, <strong>and</strong> lower isocyanate requirements<br />
compared to other polyols, such as polyether <strong>and</strong> polyester<br />
polyols. 282 A library <strong>of</strong> acrylic polyols was synthesized using a<br />
batch reactor system <strong>and</strong> conventional solution free-radical<br />
polymerization. 283 The acrylic monomers utilized were 2-hydroxyethyl<br />
acrylate (HEA), butyl methacrylate (BMA), <strong>and</strong> n-butyl<br />
acrylate (BA). The library <strong>of</strong> acrylic polyols varied with respect to<br />
HEA content <strong>and</strong> BA/BMA ratio. A total <strong>of</strong> 24 different acrylic<br />
polyols were produced in a single run. The polyols were<br />
characterized using high-throughput methods for determining<br />
Tg, relative molecular weight, <strong>and</strong> molecular weight distribution.<br />
As expected, at a constant HEA repeat unit content, acrylic polyol<br />
Tg increased with decreasing BA/BMA ratio. At a constant<br />
606 dx.doi.org/10.1021/co200007w |ACS Comb. Sci. 2011, 13, 579–633