Combinatorial and High-Throughput Screening of Materials ...
Combinatorial and High-Throughput Screening of Materials ...
Combinatorial and High-Throughput Screening of Materials ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
ACS <strong>Combinatorial</strong> Science<br />
REVIEW<br />
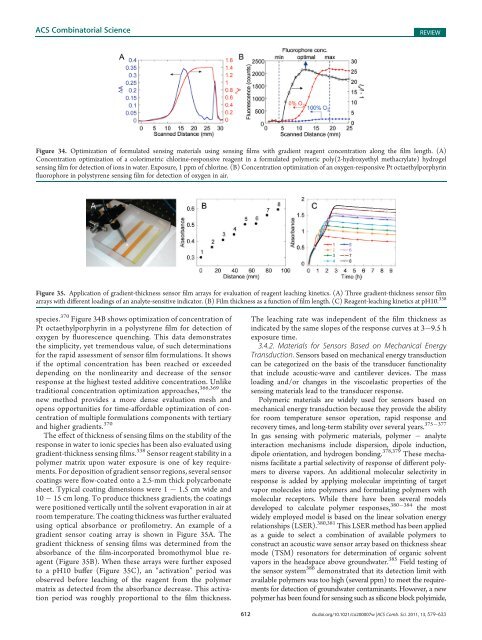
Figure 34. Optimization <strong>of</strong> formulated sensing materials using sensing films with gradient reagent concentration along the film length. (A)<br />
Concentration optimization <strong>of</strong> a colorimetric chlorine-responsive reagent in a formulated polymeric poly(2-hydroxyethyl methacrylate) hydrogel<br />
sensing film for detection <strong>of</strong> ions in water. Exposure, 1 ppm <strong>of</strong> chlorine. (B) Concentration optimization <strong>of</strong> an oxygen-responsive Pt octaethylporphyrin<br />
fluorophore in polystyrene sensing film for detection <strong>of</strong> oxygen in air.<br />
Figure 35. Application <strong>of</strong> gradient-thickness sensor film arrays for evaluation <strong>of</strong> reagent leaching kinetics. (A) Three gradient-thickness sensor film<br />
arrays with different loadings <strong>of</strong> an analyte-sensitive indicator. (B) Film thickness as a function <strong>of</strong> film length. (C) Reagent-leaching kinetics at pH10. 338<br />
species. 370 Figure 34B shows optimization <strong>of</strong> concentration <strong>of</strong><br />
Pt octaethylporphyrin in a polystyrene film for detection <strong>of</strong><br />
oxygen by fluorescence quenching. This data demonstrates<br />
the simplicity, yet tremendous value, <strong>of</strong> such determinations<br />
for the rapid assessment <strong>of</strong> sensor film formulations. It shows<br />
if the optimal concentration has been reached or exceeded<br />
depending on the nonlinearity <strong>and</strong> decrease <strong>of</strong> the sensor<br />
response at the highest tested additive concentration. Unlike<br />
traditional concentration optimization approaches, 366,369 the<br />
new method provides a more dense evaluation mesh <strong>and</strong><br />
opens opportunities for time-affordable optimization <strong>of</strong> concentration<br />
<strong>of</strong> multiple formulations components with tertiary<br />
<strong>and</strong> higher gradients. 370<br />
The effect <strong>of</strong> thickness <strong>of</strong> sensing films on the stability <strong>of</strong> the<br />
response in water to ionic species has been also evaluated using<br />
gradient-thickness sensing films. 338 Sensor reagent stability in a<br />
polymer matrix upon water exposure is one <strong>of</strong> key requirements.<br />
For deposition <strong>of</strong> gradient sensor regions, several sensor<br />
coatings were flow-coated onto a 2.5-mm thick polycarbonate<br />
sheet. Typical coating dimensionswere1 1.5 cm wide <strong>and</strong><br />
10 15 cm long. To produce thickness gradients, the coatings<br />
were positioned vertically until the solvent evaporation in air at<br />
room temperature. The coating thickness was further evaluated<br />
using optical absorbance or pr<strong>of</strong>ilometry. An example <strong>of</strong> a<br />
gradient sensor coating array is shown in Figure 35A. The<br />
gradient thickness <strong>of</strong> sensing films was determined from the<br />
absorbance <strong>of</strong> the film-incorporated bromothymol blue reagent<br />
(Figure 35B). When these arrays were further exposed<br />
to a pH10 buffer (Figure 35C), an “activation” period was<br />
observed before leaching <strong>of</strong> the reagent from the polymer<br />
matrix as detected from the absorbance decrease. This activation<br />
period was roughly proportional to the film thickness.<br />
The leaching rate was independent <strong>of</strong> the film thickness as<br />
indicated by the same slopes <strong>of</strong> the response curves at 3 9.5 h<br />
exposure time.<br />
3.4.2. <strong>Materials</strong> for Sensors Based on Mechanical Energy<br />
Transduction. Sensors based on mechanical energy transduction<br />
can be categorized on the basis <strong>of</strong> the transducer functionality<br />
that include acoustic-wave <strong>and</strong> cantilever devices. The mass<br />
loading <strong>and</strong>/or changes in the viscoelastic properties <strong>of</strong> the<br />
sensing materials lead to the transducer response.<br />
Polymeric materials are widely used for sensors based on<br />
mechanical energy transduction because they provide the ability<br />
for room temperature sensor operation, rapid response <strong>and</strong><br />
375 377<br />
recovery times, <strong>and</strong> long-term stability over several years.<br />
In gas sensing with polymeric materials, polymer analyte<br />
interaction mechanisms include dispersion, dipole induction,<br />
dipole orientation, <strong>and</strong> hydrogen bonding. 378,379 These mechanisms<br />
facilitate a partial selectivity <strong>of</strong> response <strong>of</strong> different polymers<br />
to diverse vapors. An additional molecular selectivity in<br />
response is added by applying molecular imprinting <strong>of</strong> target<br />
vapor molecules into polymers <strong>and</strong> formulating polymers with<br />
molecular receptors. While there have been several models<br />
developed to calculate polymer responses, 380 384 the most<br />
widely employed model is based on the linear solvation energy<br />
relationships (LSER). 380,381 This LSER method has been applied<br />
as a guide to select a combination <strong>of</strong> available polymers to<br />
construct an acoustic wave sensor array based on thickness shear<br />
mode (TSM) resonators for determination <strong>of</strong> organic solvent<br />
vapors in the headspace above groundwater. 385 Field testing <strong>of</strong><br />
the sensor system 386 demonstrated that its detection limit with<br />
available polymers was too high (several ppm) to meet the requirements<br />
for detection <strong>of</strong> groundwater contaminants. However, a new<br />
polymer has been found for sensing such as silicone block polyimide,<br />
612 dx.doi.org/10.1021/co200007w |ACS Comb. Sci. 2011, 13, 579–633