Chem 55 Exam 2 Solutions
Chem 55 Exam 2 Solutions
Chem 55 Exam 2 Solutions
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
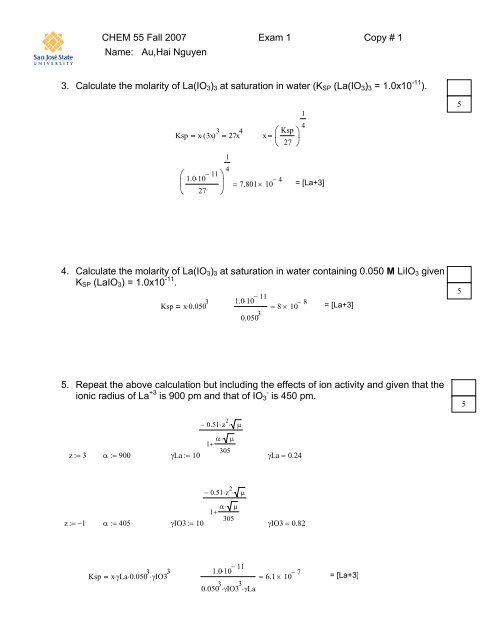
CHEM <strong>55</strong> Fall 2007 <strong>Exam</strong> 1 Copy # 1<br />
Name: Au,Hai Nguyen<br />
3. Calculate the molarity of La(IO 3 ) 3 at saturation in water (K SP (La(IO 3 ) 3 = 1.0x10 -11 ).<br />
Ksp<br />
⎛<br />
⎜<br />
⎝<br />
x⋅ ( 3x) 3 27x 4 x<br />
1.0⋅<br />
10 − 11<br />
27<br />
⎞<br />
⎟<br />
⎠<br />
1<br />
4<br />
⎛<br />
⎜<br />
⎝<br />
Ksp<br />
27<br />
⎞ ⎟⎠<br />
1<br />
4<br />
= 7.801×<br />
10 − 4 = [La+3]<br />
5<br />
4. Calculate the molarity of La(IO 3 ) 3 at saturation in water containing 0.050 M LiIO 3 given<br />
K SP (LaIO 3 ) = 1.0x10 -11 .<br />
Ksp x⋅<br />
0.050 3<br />
1.0⋅<br />
10 − 11<br />
= 8×<br />
10 − 8 = [La+3]<br />
0.050 3<br />
5<br />
5. Repeat the above calculation but including the effects of ion activity and given that the<br />
ionic radius of La +3 is 900 pm and that of IO 3 - is 450 pm.<br />
5<br />
− 0.51⋅z 2 ⋅ μ<br />
1+<br />
α⋅ μ<br />
305<br />
z:= 3 α := 900<br />
γLa := 10<br />
γLa = 0.24<br />
− 0.51⋅z 2 ⋅ μ<br />
1+<br />
α⋅ μ<br />
305<br />
z:= −1<br />
α := 405<br />
γIO3 := 10<br />
γIO3 = 0.82<br />
Ksp<br />
x⋅ γLa⋅0.050 3 ⋅γIO3 3<br />
1.0⋅<br />
10 − 11<br />
0.050 3 ⋅γIO3 3 ⋅γLa<br />
= 6.1 × 10 − 7 = [La+3]