Chem 55 Exam 2 Solutions
Chem 55 Exam 2 Solutions
Chem 55 Exam 2 Solutions
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
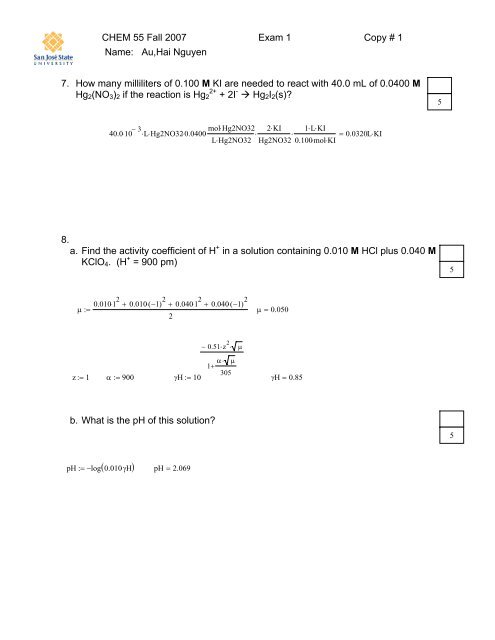
CHEM <strong>55</strong> Fall 2007 <strong>Exam</strong> 1 Copy # 1<br />
Name: Au,Hai Nguyen<br />
7. How many milliliters of 0.100 M KI are needed to react with 40.0 mL of 0.0400 M<br />
Hg 2 (NO 3 ) 2 if the reaction is Hg 2 2+ + 2I - Hg 2 I 2 (s)<br />
5<br />
40.0⋅<br />
10 − 3<br />
mol⋅<br />
Hg2NO32 2KI ⋅ 1L ⋅ ⋅KI<br />
⋅L⋅ Hg2NO32⋅<br />
0.0400⋅<br />
⋅ ⋅<br />
=<br />
L⋅<br />
Hg2NO32 Hg2NO32 0.100⋅<br />
mol⋅<br />
KI<br />
0.0320L⋅<br />
KI<br />
8.<br />
a. Find the activity coefficient of H + in a solution containing 0.010 M HCl plus 0.040 M<br />
KClO 4 . (H + = 900 pm)<br />
5<br />
0.010⋅ 1 2 + 0.010⋅( −1) 2 + 0.040⋅<br />
1 2 + 0.040⋅( −1) 2<br />
μ := μ = 0.050<br />
2<br />
− 0.51⋅z 2 ⋅<br />
1+<br />
α⋅<br />
μ<br />
305<br />
z:= 1 α := 900<br />
γH:= 10<br />
γH = 0.85<br />
μ<br />
b. What is the pH of this solution<br />
5<br />
( )<br />
pH := −log 0.010⋅<br />
γH pH = 2.069