Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
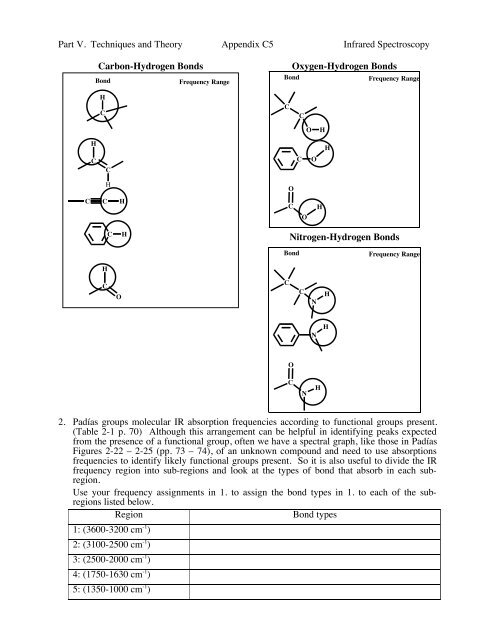
Part V. Techniques and Theory Appendix C5 Infrared Spectroscopy<br />
Carbon-Hydrogen Bonds<br />
Bond<br />
Frequency Range<br />
Bond<br />
Oxygen-Hydrogen Bonds<br />
Frequency Range<br />
H<br />
C<br />
C<br />
C<br />
O<br />
H<br />
H<br />
H<br />
C<br />
C<br />
C<br />
O<br />
H<br />
O<br />
C C H<br />
C<br />
O<br />
H<br />
C<br />
H<br />
Nitrogen-Hydrogen Bonds<br />
Bond<br />
Frequency Range<br />
H<br />
C<br />
O<br />
C<br />
C<br />
N<br />
H<br />
N<br />
H<br />
O<br />
C<br />
N<br />
H<br />
2. Padías groups molecular IR absorption frequencies according to functional groups present.<br />
(Table 2-1 p. 70) Although this arrangement can be helpful in identifying peaks expected<br />
from the presence of a functional group, often we have a spectral graph, like those in Padías<br />
Figures 2-22 – 2-25 (pp. 73 – 74), of an unknown compound and need to use absorptions<br />
frequencies to identify likely functional groups present. So it is also useful to divide the IR<br />
frequency region into sub-regions and look at the types of bond that absorb in each subregion.<br />
Use your frequency assignments in 1. to assign the bond types in 1. to each of the subregions<br />
listed below.<br />
Region<br />
Bond types<br />
1: (3600-3200 cm -1 )<br />
2: (3100-2500 cm -1 )<br />
3: (2500-2000 cm -1 )<br />
4: (1750-1630 cm -1 )<br />
5: (1350-1000 cm -1 )