Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
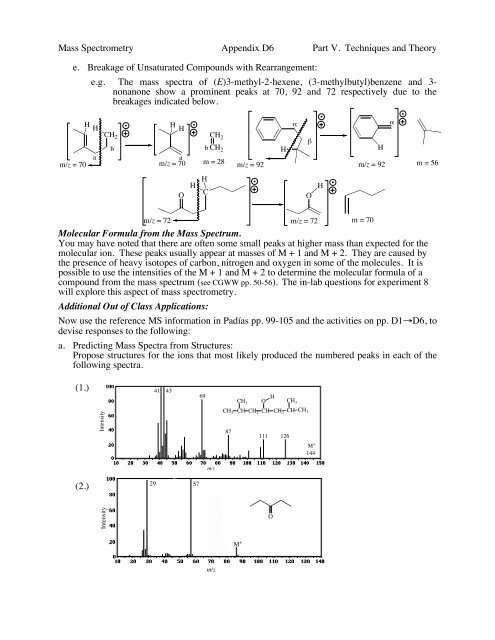
Mass Spectrometry Appendix D6 Part V. Techniques and Theory<br />
e. Breakage of Unsaturated Compounds with Rearrangement:<br />
e.g. The mass spectra of (E)3-methyl-2-hexene, (3-methylbutyl)benzene and 3-<br />
nonanone show a prominent peaks at 70, 92 and 72 respectively due to the<br />
breakages indicated below.<br />
H<br />
H<br />
a<br />
m/z = 70<br />
CH 2<br />
b<br />
H<br />
H<br />
a<br />
m/z = 70<br />
CH 2<br />
b CH 2<br />
m = 28 m/z = 92<br />
H<br />
"<br />
!<br />
H<br />
"<br />
m/z = 92 m = 56<br />
O<br />
H<br />
H<br />
C<br />
O<br />
H<br />
m/z = 72<br />
m/z = 72 m = 70<br />
Molecular Formula from the Mass Spectrum.<br />
You may have noted that there are often some small peaks at higher mass than expected for the<br />
molecular ion. These peaks usually appear at masses of M + 1 and M + 2. They are caused by<br />
the presence of heavy isotopes of carbon, nitrogen and oxygen in some of the molecules. It is<br />
possible to use the intensities of the M + 1 and M + 2 to determine the molecular formula of a<br />
compound from the mass spectrum (see CGWW pp. 50-56). The in-lab questions for experiment 8<br />
will explore this aspect of mass spectrometry.<br />
Additional Out of Class Applications:<br />
Now use the reference MS information in Padías pp. 99-105 and the activities on pp. D1→D6, to<br />
devise responses to the following:<br />
a. Predicting Mass Spectra from Structures:<br />
Propose structures for the ions that most likely produced the numbered peaks in each of the<br />
following spectra.<br />
(1.)<br />
Intensity<br />
41<br />
43<br />
69<br />
CH 3<br />
87<br />
H<br />
CH 3 O<br />
CH CH 2 CH<br />
111<br />
CH 3<br />
CH 2<br />
CH CH 3<br />
126<br />
M +<br />
144<br />
m/z<br />
(2.)<br />
29<br />
57<br />
Intensity<br />
O<br />
M +<br />
m/z