Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
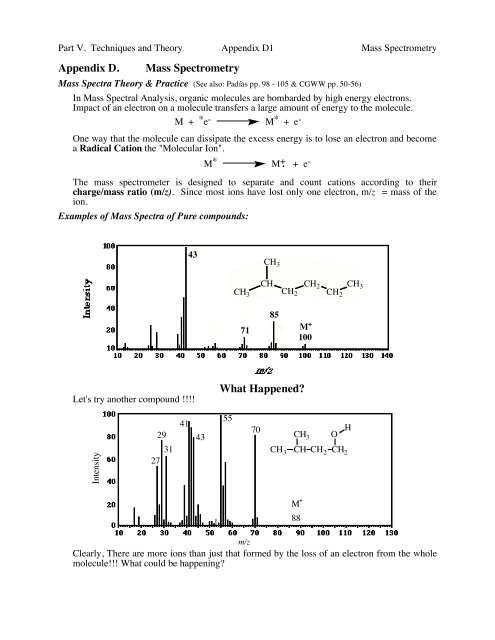
Part V. Techniques and Theory Appendix D1 Mass Spectrometry<br />
Appendix D.<br />
Mass Spectrometry<br />
Mass Spectra Theory & Practice (See also: Padías pp. 98 - 105 & CGWW pp. 50-56)<br />
In Mass Spectral Analysis, organic molecules are bombarded by high energy electrons.<br />
Impact of an electron on a molecule transfers a large amount of energy to the molecule.<br />
M + * e - M * + e -<br />
One way that the molecule can dissipate the excess energy is to lose an electron and become<br />
a Radical Cation the "Molecular Ion".<br />
M * M + . + e -<br />
The mass spectrometer is designed to separate and count cations according to their<br />
charge/mass ratio (m/z). Since most ions have lost only one electron, m/z = mass of the<br />
ion.<br />
Examples of Mass Spectra of Pure compounds:<br />
43<br />
CH 3<br />
CH<br />
CH 3 CH 2<br />
CH 2<br />
CH2<br />
CH 3<br />
71<br />
85<br />
M +<br />
100<br />
Let's try another compound !!!!<br />
What Happened<br />
Intensity<br />
27<br />
29<br />
31<br />
41<br />
43<br />
55<br />
70<br />
CH 3<br />
CH 3 O<br />
H<br />
CH CH 2 CH 2<br />
M +<br />
88<br />
m/z<br />
Clearly, There are more ions than just that formed by the loss of an electron from the whole<br />
molecule!!! What could be happening