Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
Organic Chemistry Semester 1 LABORATORY MANUAL - Moravian ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Part V. Techniques and Theory Appendix E19 Nuclear Magnetic Resonance<br />
d. Examples:<br />
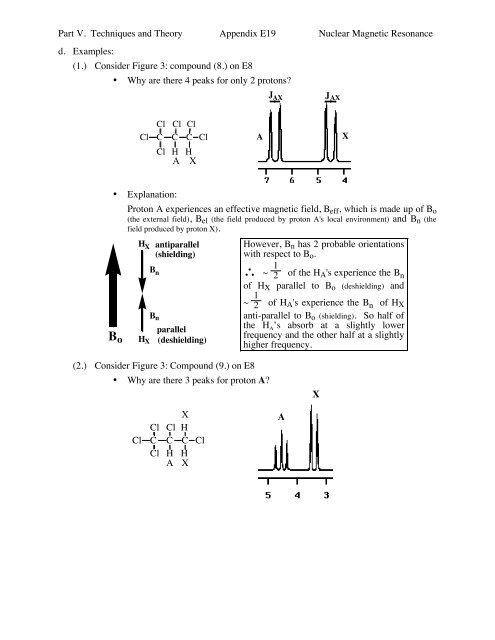
(1.) Consider Figure 3: compound (8.) on E8<br />
• Why are there 4 peaks for only 2 protons<br />
J AX<br />
J AX<br />
Cl Cl Cl<br />
Cl C C C Cl<br />
Cl H H<br />
A X<br />
A<br />
X<br />
• Explanation:<br />
B o<br />
Proton A experiences an effective magnetic field, B eff , which is made up of B o<br />
(the external field), B el (the field produced by proton A's local environment) and B n (the<br />
field produced by proton X).<br />
H X<br />
H X<br />
antiparallel<br />
(shielding)<br />
B n<br />
B n<br />
parallel<br />
(deshielding)<br />
However, B n has 2 probable orientations<br />
with respect to B o .<br />
∴ ~ 1<br />
2 of the H A's experience the B n<br />
of H X parallel to B o (deshielding) and<br />
~ 1<br />
2 of H A's experience the B n of H X<br />
anti-parallel to B o (shielding). So half of<br />
the H A ’s absorb at a slightly lower<br />
frequency and the other half at a slightly<br />
higher frequency.<br />
(2.) Consider Figure 3: Compound (9.) on E8<br />
• Why are there 3 peaks for proton A<br />
X<br />
X<br />
Cl<br />
Cl<br />
C<br />
Cl<br />
C<br />
H<br />
C<br />
Cl H H<br />
A X<br />
Cl<br />
A