P - Technische Universiteit Eindhoven
P - Technische Universiteit Eindhoven
P - Technische Universiteit Eindhoven
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2. Single crystal growth of sodium sulfate heptahydrate 19<br />
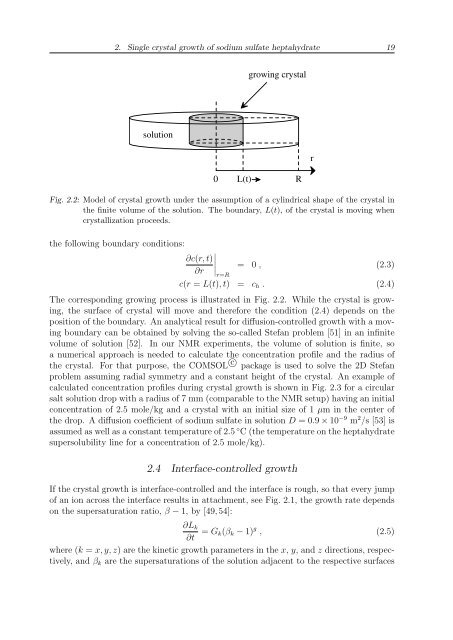
growing crystal<br />
solution<br />
r<br />
0 L(t)<br />
R<br />
Fig. 2.2: Model of crystal growth under the assumption of a cylindrical shape of the crystal in<br />
the finite volume of the solution. The boundary, L(t), of the crystal is moving when<br />
crystallization proceeds.<br />
the following boundary conditions:<br />
∂c(r, t)<br />
∂r ∣ r=R<br />
= 0 , (2.3)<br />
c(r = L(t), t) = c h . (2.4)<br />
The corresponding growing process is illustrated in Fig. 2.2. While the crystal is growing,<br />
the surface of crystal will move and therefore the condition (2.4) depends on the<br />
position of the boundary. An analytical result for diffusion-controlled growth with a moving<br />
boundary can be obtained by solving the so-called Stefan problem [51] in an infinite<br />
volume of solution [52]. In our NMR experiments, the volume of solution is finite, so<br />
a numerical approach is needed to calculate the concentration profile and the radius of<br />
the crystal. For that purpose, the COMSOL c⃝ package is used to solve the 2D Stefan<br />
problem assuming radial symmetry and a constant height of the crystal. An example of<br />
calculated concentration profiles during crystal growth is shown in Fig. 2.3 for a circular<br />
salt solution drop with a radius of 7 mm (comparable to the NMR setup) having an initial<br />
concentration of 2.5 mole/kg and a crystal with an initial size of 1 µm in the center of<br />
the drop. A diffusion coefficient of sodium sulfate in solution D = 0.9 × 10 −9 m 2 /s [53] is<br />
assumed as well as a constant temperature of 2.5 ◦ C (the temperature on the heptahydrate<br />
supersolubility line for a concentration of 2.5 mole/kg).<br />
2.4 Interface-controlled growth<br />
If the crystal growth is interface-controlled and the interface is rough, so that every jump<br />
of an ion across the interface results in attachment, see Fig. 2.1, the growth rate depends<br />
on the supersaturation ratio, β − 1, by [49, 54]:<br />
∂L k<br />
= G k (β k − 1) g , (2.5)<br />
∂t<br />
where (k = x, y, z) are the kinetic growth parameters in the x, y, and z directions, respectively,<br />
and β k are the supersaturations of the solution adjacent to the respective surfaces