P - Technische Universiteit Eindhoven
P - Technische Universiteit Eindhoven
P - Technische Universiteit Eindhoven
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3. Crystallization under wetting/non-wetting conditions 36<br />
A<br />
thermocouple<br />
B<br />
droplet-surface<br />
interface<br />
14 mm<br />
droplet<br />
LED<br />
illumination<br />
moon-shaped<br />
droplet<br />
C<br />
D<br />
initial<br />
heptahydrate<br />
crystal<br />
formed<br />
heptahydrate<br />
crystal<br />
E<br />
dehydrating<br />
heptahydrate<br />
dehydration<br />
front<br />
F<br />
thenardite<br />
heptahydrate covered<br />
with thernadite<br />
forming<br />
anhydrous<br />
thernadite<br />
thernadite<br />
covering<br />
heptahydrate<br />
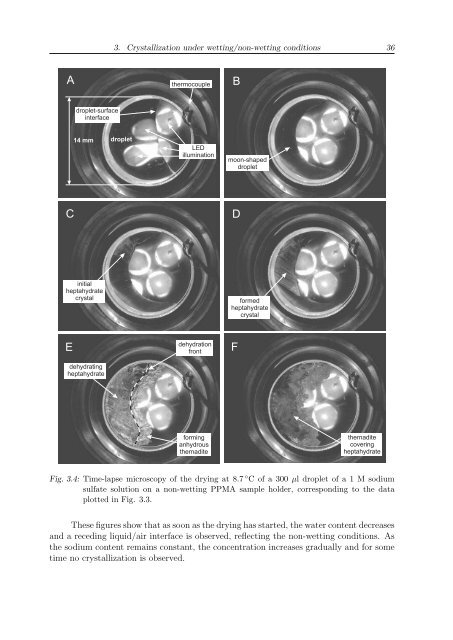
Fig. 3.4: Time-lapse microscopy of the drying at 8.7 ◦ C of a 300 µl droplet of a 1 M sodium<br />
sulfate solution on a non-wetting PPMA sample holder, corresponding to the data<br />
plotted in Fig. 3.3.<br />
These figures show that as soon as the drying has started, the water content decreases<br />
and a receding liquid/air interface is observed, reflecting the non-wetting conditions. As<br />
the sodium content remains constant, the concentration increases gradually and for some<br />
time no crystallization is observed.