P - Technische Universiteit Eindhoven
P - Technische Universiteit Eindhoven
P - Technische Universiteit Eindhoven
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
7. Thermodynamic and poromechanic crystallization pressure of sodium sulfate heptahydrate 76<br />
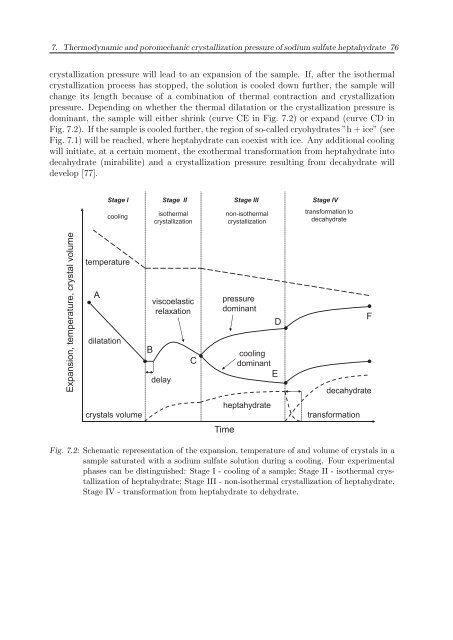
crystallization pressure will lead to an expansion of the sample. If, after the isothermal<br />
crystallization process has stopped, the solution is cooled down further, the sample will<br />
change its length because of a combination of thermal contraction and crystallization<br />
pressure. Depending on whether the thermal dilatation or the crystallization pressure is<br />
dominant, the sample will either shrink (curve CE in Fig. 7.2) or expand (curve CD in<br />
Fig. 7.2). If the sample is cooled further, the region of so-called cryohydrates ”h + ice” (see<br />
Fig. 7.1) will be reached, where heptahydrate can coexist with ice. Any additional cooling<br />
will initiate, at a certain moment, the exothermal transformation from heptahydrate into<br />
decahydrate (mirabilite) and a crystallization pressure resulting from decahydrate will<br />
develop [77].<br />
Stage I Stage II Stage III<br />
cooling<br />
isothermal<br />
crystallization<br />
non-isothermal<br />
crystallization<br />
Stage IV<br />
transformation to<br />
decahydrate<br />
Expansion, temperature, crystal volume<br />
temperature<br />
A<br />
dilatation<br />
B<br />
viscoelastic<br />
relaxation<br />
delay<br />
C<br />
pressure<br />
dominant<br />
D<br />
cooling<br />
dominant<br />
E<br />
F<br />
decahydrate<br />
crystals volume<br />
heptahydrate<br />
transformation<br />
Time<br />
Fig. 7.2: Schematic representation of the expansion, temperature of and volume of crystals in a<br />
sample saturated with a sodium sulfate solution during a cooling. Four experimental<br />
phases can be distinguished: Stage I - cooling of a sample; Stage II - isothermal crystallization<br />
of heptahydrate; Stage III - non-isothermal crystallization of heptahydrate,<br />
Stage IV - transformation from heptahydrate to dehydrate.