P - Technische Universiteit Eindhoven
P - Technische Universiteit Eindhoven
P - Technische Universiteit Eindhoven
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3. Crystallization under wetting/non-wetting conditions 39<br />
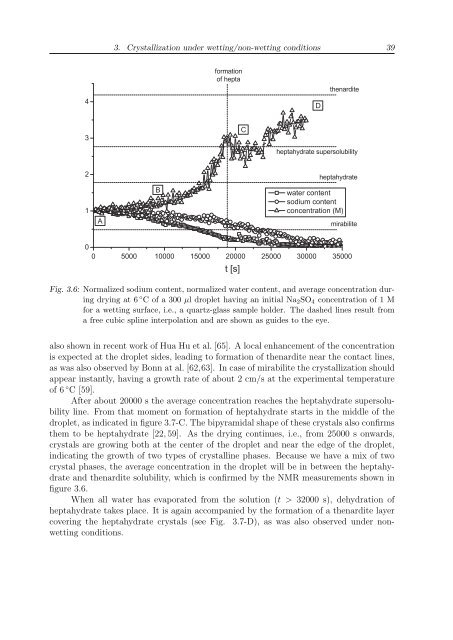
Fig. 3.6: Normalized sodium content, normalized water content, and average concentration during<br />
drying at 6 ◦ C of a 300 µl droplet having an initial Na 2 SO 4 concentration of 1 M<br />
for a wetting surface, i.e., a quartz-glass sample holder. The dashed lines result from<br />
a free cubic spline interpolation and are shown as guides to the eye.<br />
t [s]<br />
also shown in recent work of Hua Hu et al. [65]. A local enhancement of the concentration<br />
is expected at the droplet sides, leading to formation of thenardite near the contact lines,<br />
as was also observed by Bonn at al. [62,63]. In case of mirabilite the crystallization should<br />
appear instantly, having a growth rate of about 2 cm/s at the experimental temperature<br />
of 6 ◦ C [59].<br />
After about 20000 s the average concentration reaches the heptahydrate supersolubility<br />
line. From that moment on formation of heptahydrate starts in the middle of the<br />
droplet, as indicated in figure 3.7-C. The bipyramidal shape of these crystals also confirms<br />
them to be heptahydrate [22, 59]. As the drying continues, i.e., from 25000 s onwards,<br />
crystals are growing both at the center of the droplet and near the edge of the droplet,<br />
indicating the growth of two types of crystalline phases. Because we have a mix of two<br />
crystal phases, the average concentration in the droplet will be in between the heptahydrate<br />
and thenardite solubility, which is confirmed by the NMR measurements shown in<br />
figure 3.6.<br />
When all water has evaporated from the solution (t > 32000 s), dehydration of<br />
heptahydrate takes place. It is again accompanied by the formation of a thenardite layer<br />
covering the heptahydrate crystals (see Fig. 3.7-D), as was also observed under nonwetting<br />
conditions.