P - Technische Universiteit Eindhoven
P - Technische Universiteit Eindhoven
P - Technische Universiteit Eindhoven
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
4. Nucleation on mineral substrates 47<br />
c [mole/kg]<br />
time [s]<br />
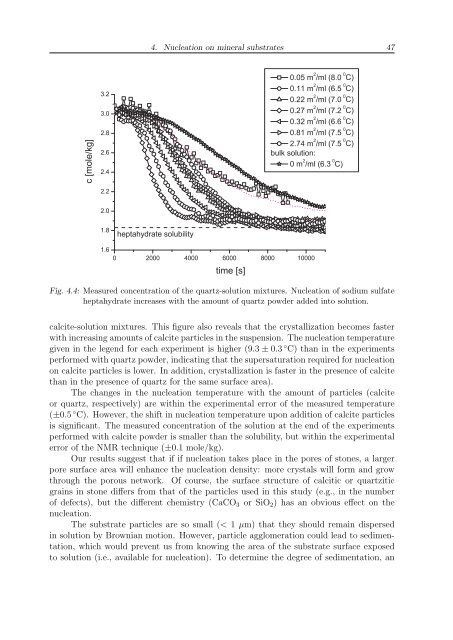
Fig. 4.4: Measured concentration of the quartz-solution mixtures. Nucleation of sodium sulfate<br />
heptahydrate increases with the amount of quartz powder added into solution.<br />
calcite-solution mixtures. This figure also reveals that the crystallization becomes faster<br />
with increasing amounts of calcite particles in the suspension. The nucleation temperature<br />
given in the legend for each experiment is higher (9.3 ± 0.3 ◦ C) than in the experiments<br />
performed with quartz powder, indicating that the supersaturation required for nucleation<br />
on calcite particles is lower. In addition, crystallization is faster in the presence of calcite<br />
than in the presence of quartz for the same surface area).<br />
The changes in the nucleation temperature with the amount of particles (calcite<br />
or quartz, respectively) are within the experimental error of the measured temperature<br />
(±0.5 ◦ C). However, the shift in nucleation temperature upon addition of calcite particles<br />
is significant. The measured concentration of the solution at the end of the experiments<br />
performed with calcite powder is smaller than the solubility, but within the experimental<br />
error of the NMR technique (±0.1 mole/kg).<br />
Our results suggest that if if nucleation takes place in the pores of stones, a larger<br />
pore surface area will enhance the nucleation density: more crystals will form and grow<br />
through the porous network. Of course, the surface structure of calcitic or quartzitic<br />
grains in stone differs from that of the particles used in this study (e.g., in the number<br />
of defects), but the different chemistry (CaCO 3 or SiO 2 ) has an obvious effect on the<br />
nucleation.<br />
The substrate particles are so small (< 1 µm) that they should remain dispersed<br />
in solution by Brownian motion. However, particle agglomeration could lead to sedimentation,<br />
which would prevent us from knowing the area of the substrate surface exposed<br />
to solution (i.e., available for nucleation). To determine the degree of sedimentation, an