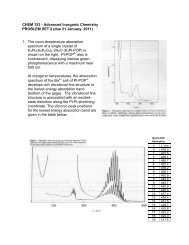
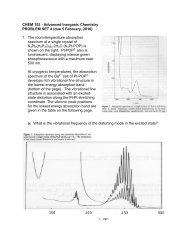
Problem Set 3
Problem Set 3
Problem Set 3
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
and [½, ½, ½] in the coordinate system with fourfold quantization, what are their positions in<br />
the coordinate system with threefold quantization<br />
b. Use the real d‐orbital functions to find basis sets for the t 2 and e representations of the T d point<br />
group in the coordinate system with trigonal quantization.<br />
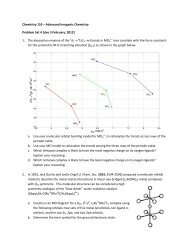
3. In 1993, Wilkinson and Hursthouse reported<br />
(Polyhedron, 1993, 12, 2009‐2012) the preparation<br />
and X‐ray crystal structure of Ir(O)(mes)3 (mes =<br />
mesityl, 2,4,6‐trimethylphenyl). The structure of the<br />
complex is shown to the right.<br />
a. Construct an MO diagram for C 3v Ir(O)(mes) 3<br />
using the following orbitals: five metal 5d orbitals, one set of three ligand σ orbitals, and the oxo<br />
2pσ + two 2pπ orbitals.<br />
b. Extend your diagram to illustrate the correlation between the trigonally quantized ML 4 orbitals<br />
and the MOs of Ir(O)(mes) 3 .<br />
c. Is there a unique set of d‐orbitals in C 3v symmetry that forms a basis for the e irreducible<br />
representation If so, what is that set of d‐orbitals If not, what is the implication for the<br />
molecular orbitals of e symmetry in Ir(O)(mes) 3 <br />
d. Using your MO diagram, fill in the d electrons and predict the ground‐state electronic<br />
configuration. With this prediction, is the metal‐oxo bond order shown above correct Why or<br />
why not Do you think that a complex of the form [Ir(O)(mes) 3 ] 2− could exist Why or why not<br />
e. Does Ir(O)(mes) 3 represent a breach of the oxo wall Why or why not