CHEM 153 - Advanced Inorganic Chemistry PROBLEM SET 3 (due ...
CHEM 153 - Advanced Inorganic Chemistry PROBLEM SET 3 (due ...
CHEM 153 - Advanced Inorganic Chemistry PROBLEM SET 3 (due ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>CHEM</strong> <strong>153</strong> - <strong>Advanced</strong> <strong>Inorganic</strong> <strong>Chemistry</strong><br />
<strong>PROBLEM</strong> <strong>SET</strong> 3 (<strong>due</strong> 28 January, 2011)<br />
1. Consider the following oxo complexes:<br />
a. [L 5 VO] 2+ V IV d 1<br />
b. [L 5 CrO] 3+ Cr V d 1<br />
c. [L 5 CrO] 2+ Cr IV d 2<br />
d. [L 5 MnO] 3+ Mn V d 2<br />
e. [L 5 MnO] 2+ Mn IV d 3<br />
f. [L 5 FeO] 2+ Fe IV d 4<br />
Part 1:<br />
Construct a ligand-field (LF) diagram for C 4v [L 5 MO] n+ (L is an uncharged ligand,<br />
for example, H 2 O or NH 3 ) using the following orbitals: five metal 3d orbitals, one<br />
set of five ligand F orbitals, and the oxo 2pF + two 2pB orbitals.<br />
Part 2:<br />
Using your LF diagram, for each of the above examples, fill in the d electrons and<br />
predict the ground-state electronic configuration. With this prediction, calculate<br />
the metal-oxo bond order. Based on your results, do you think that a complex of<br />
the form [L 5 CoO] n+ could exist Why or why not<br />
Part 3:<br />
a. Larry Que and coworkers reported the synthesis and isolation of a nonheme<br />
ferryl complex (Science 2003, 299, 1037-1039). What are the Fe-O bond<br />
properties (bond length and bond order) Are they consistent with your<br />
predictions for 6-coordinate Fe IV How do the reported Mössbauer spectra<br />
support these findings<br />
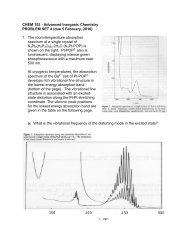
b. The electronic structures and spectroscopic properties of Que’s nonheme<br />
ferryl complex have been investigated in Ed Solomon’s laboratory (J. Am.<br />
Chem. Soc. 2004, 126, 5378-7379). An absorption system in the 10,000<br />
cm !1 region, called band I by Solomon, is assigned to the (xy)6(x 2 !y 2 )<br />
transition in the ferryl spectrum. What is the electronic configuration of the<br />
excited state formed by this transition What is the value of S (the spin<br />
quantum number) for this excited state The energies of the transitions<br />
associated with bands I and II are at much lower energies than the<br />
corresponding transitions in d 1 metal-oxo complexes such as [L 5 VO] 2+ and<br />
[L 5 CrO] 3+ . How does Solomon account for these differences
Part 4:<br />
The first example of a trans-dioxo (D 4h ) 3d metal complex was reported in 2007<br />
(J. Am. Chem. Soc. 2007, 129, 12416-12417). Construct an LF diagram (you<br />
need only show the 3d levels) for the complex. What is the electronic<br />
configuration of the ground state What is the oxo-metal bond order What are<br />
the term symbols for the two lowest electronic excited states Did the authors<br />
observe transitions from the ground state to these excited states in the<br />
absorption spectrum of the complex Should they have Explain briefly.<br />
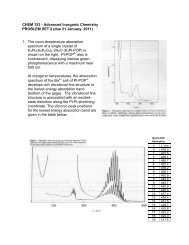
2. The electronic absorption spectra of [Cr V (N)(CN) 5 ] 3! and [Mn V (N)(CN) 5 ] 3! are shown<br />
below. In [Cr V (N)(CN) 5 ] 3! , the 2 B 2 [(xy)] ÷ 2 E[(xz,yz)] absorption band is at 23,300<br />
cm !1 . In [Mn V (N)(CN) 5 ] 3! , the 1 A 1 [(xy) 2 ] ÷ 1 E[(xy)(xz,yz)] absorption band is at<br />
19,400 cm !1 .<br />
[Cr(N)(CN) [Cr(N)(CN)5] 5 ] 3 3!<br />
[Mn(N)(CN) 5<br />
] 3!<br />
[Mn(N)(CN) 5 ] 3
Use the following orbital splitting diagram to determine the values of ) B in the Cr and<br />
Mn complexes. Expressions for the electron-electron repulsion integrals are given<br />
below. Assume that B = 500 cm !1 and that C/B = 4.<br />
B<br />
d 2 :<br />
3 A 2 [(xz,yz) 2 ] E = 2 ) B + A ! 5B<br />
1 A 1 [(xz,yz) 2 ] E = 2 ) B + A + 7B + 4C<br />
1 B 1 [(xz,yz) 2 ] E = 2 ) B + A + B + 2C<br />
1 B 2 [(xz,yz) 2 ] E = 2 ) B + A +B + 2C<br />
1 E[(xy) 1 (xz,yz) 1 ] E = ) B + A + B + 2C<br />
3 E[(xy) 1 (xz,yz) 1 ] E = ) B + A ! 5B<br />
1 A 1 [(xy) 2 ] E = A + 4B + 3C<br />
d 3 :<br />
2 E[(xz,yz) 3 ] E = 3 ) B + 3A ! 3B + 4C<br />
4 B 1 [(xy) 1 (xz,yz) 2 ] E = 2 ) B + 3A !15B<br />
2 B 1 [(xy) 1 (xz,yz) 2 ] E = 2 ) B + 3A ! 6B + 3C<br />
2 A 1 [(xy) 1 (xz,yz) 2 ] E = 2 ) B + 3A ! 6B + 3C<br />
2 B 2 [(xy) 1 (xz,yz) 2 ] E = 2 ) B + 3A + 5C<br />
2 A 2 [(xy) 1 (xz,yz) 2 ] E = 2 ) B + 3A ! 6B + 3C<br />
2 E[(xy) 2 (xz,yz) 1 ] E = ) B + 3A ! 3B + 4C<br />
3. Use the foregoing orbital splitting pattern and energy expressions in considering<br />
high-spin/low-spin equilibria in tetragonal oxo- and nitrido-metal complexes with d 2<br />
and d 3 configurations.<br />
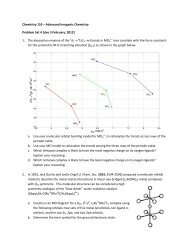
Assume that the excited-state distortion parameter 8 (as defined in the diagrams on<br />
the following page) is about 3,400 cm !1 for both high- and low-spin states.<br />
a) Find the ) B values at the high-spin/low-spin crossover points for d 2 and d 3<br />
tetragonal oxo- and nitrido-metal complexes. Assume that B = 500 cm !1 and<br />
C/B = 4.<br />
b) Assume that you have a high-spin/low-spin equilibrium in a d 2 tetragonal oxo- or
nitrido-metal complex in which E TH = 0. What are the ) B values for high- and<br />
low-spin forms<br />
c) Assume that you have a high-spin/low-spin equilibrium in a d 3 tetragonal oxoand<br />
nitrido-metal complex in which E TH = 0. What are the ) B values for high- and<br />
low-spin forms<br />
d) What are the relative populations of the high- and low-spin states in problems (b)<br />
and (c)<br />
e) Karl Wieghardt reported (Angew. Chem. Int. Ed. 2005, 44, 2908-2912) that,<br />
unexpectedly, the ground-state total spin of the [(cyclam-acetato)Fe V (N)] + core is<br />
S=1/2 and not S=3/2. On the basis of your answers to Problems 1, and 2(c),<br />
discuss whether you think that this result is “unexpected”.