Chemistry 153 â Advanced Inorganic Chemistry Problem Set 4 (due ...
Chemistry 153 â Advanced Inorganic Chemistry Problem Set 4 (due ...
Chemistry 153 â Advanced Inorganic Chemistry Problem Set 4 (due ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Chemistry</strong> <strong>153</strong> – <strong>Advanced</strong> <strong>Inorganic</strong> <strong>Chemistry</strong><br />
<strong>Problem</strong> <strong>Set</strong> 4 (<strong>due</strong> 3 February, 2012)<br />
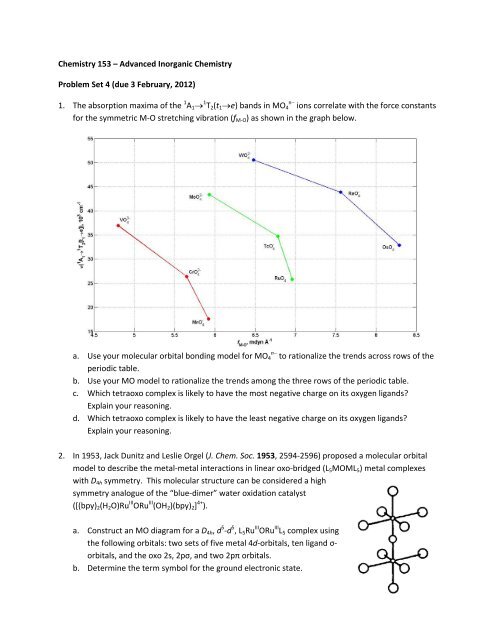
1. The absorption maxima of the 1 A 1 1 T 2 (t 1 e) bands in MO 4 n ions correlate with the force constants<br />
for the symmetric M‐O stretching vibration (f M‐O ) as shown in the graph below.<br />
a. Use your molecular orbital bonding model for MO 4 n to rationalize the trends across rows of the<br />
periodic table.<br />
b. Use your MO model to rationalize the trends among the three rows of the periodic table.<br />
c. Which tetraoxo complex is likely to have the most negative charge on its oxygen ligands<br />
Explain your reasoning.<br />
d. Which tetraoxo complex is likely to have the least negative charge on its oxygen ligands<br />
Explain your reasoning.<br />
2. In 1953, Jack Dunitz and Leslie Orgel (J. Chem. Soc. 1953, 2594‐2596) proposed a molecular orbital<br />
model to describe the metal‐metal interactions in linear oxo‐bridged (L 5 MOML 5 ) metal complexes<br />
with D 4h symmetry. This molecular structure can be considered a high<br />
symmetry analogue of the “blue‐dimer” water oxidation catalyst<br />
([(bpy) 2 (H 2 O)Ru III ORu III (OH 2 )(bpy) 2 ] 4+ ).<br />
a. Construct an MO diagram for a D 4h , d 5 ‐d 5 , L 5 Ru III ORu III L 5 complex using<br />
the following orbitals: two sets of five metal 4d‐orbitals, ten ligand σ‐<br />
orbitals, and the oxo 2s, 2pσ, and two 2pπ orbitals.<br />
b. Determine the term symbol for the ground electronic state.
c. The temperature dependence of the<br />
magnetic susceptibility and magnetic<br />
moment of the blue dimer are shown to the<br />
right. Does the magnetic behavior of this<br />
molecule agree with the prediction based on<br />
your MO model If not, propose a<br />
refinement to your model that will rationalize<br />
the discrepancies.