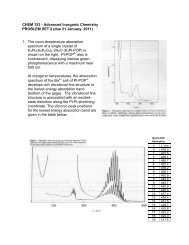
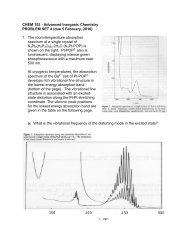
Problem Set 3
Problem Set 3
Problem Set 3
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chemistry 153 – Advanced Inorganic Chemistry<br />
<strong>Problem</strong> <strong>Set</strong> 3 (due 27 January, 2012)<br />
1. Consider the following oxo complexes:<br />
[L 5 VO] 2+ V IV d 1<br />
[L 5 CrO] 3+ Cr V d 1<br />
[L 5 CrO] 2+ Cr IV d 2<br />
[L 5 MnO] 3+ Mn V d 2<br />
[L 5 MnO] 2+ Mn IV d 3<br />
[L 5 FeO] 2+ Fe IV d 4<br />
a. Construct an MO diagram for C 4v [L 5 MO] n+ (L is an uncharged ligand, for example, H 2 O or NH 3 )<br />
using the following orbitals: five metal 3d orbitals, one set of five ligand σ orbitals, and the oxo<br />
2pσ + two 2pπ orbitals.<br />
b. Using your MO diagram, for each of the above examples, fill in the d electrons and predict the<br />
ground‐state electronic configuration. With this prediction, calculate the metal‐oxo bond order.<br />
Based on your results, do you think that a complex of the form [L 5 CoO] n+ could exist Why or<br />
why not<br />
2. The atomic d‐orbitals in tetrahedral (T d ) symmetry transform as the irreducible representations t 2<br />
and e. When the z‐axis is chosen to coincide with the S 4 axis of this point group, the standard real d‐<br />
orbital functions form basis sets for t 2 and e representations as:<br />
d<br />
2<br />
z<br />
~<br />
e: <br />
2<br />
d<br />
x<br />
(2z<br />
2<br />
x y<br />
2 2<br />
( x y<br />
1 2<br />
2<br />
2<br />
3<br />
2<br />
y<br />
~<br />
2<br />
)<br />
<br />
<br />
d<br />
)<br />
<br />
; t 2 : d<br />
<br />
d<br />
xz<br />
yz<br />
xy<br />
~<br />
~<br />
~<br />
3xz<br />
<br />
3yz<br />
<br />
3xy<br />
<br />
<br />
This set of functions is well‐adapted to molecules of tetragonal symmetry, but is less convenient for<br />
molecules of trigonal symmetry. An alternative approach to the description of molecules of trigonal<br />
symmetry could begin with the T d point group but with the z‐axis coincident with the threefold<br />
rotation axis. This coordinate transformation can be accomplished with a 45 rotation about the z‐<br />
axis followed by a 54.7 rotation about the new x‐axis. Defining the original coordinate system as<br />
x,y,z, and the new coordinate system as X,Y,Z, leads to the following transformations:<br />
<br />
X<br />
<br />
<br />
Y<br />
<br />
<br />
<br />
<br />
Z<br />
<br />
<br />
1<br />
2<br />
1<br />
6<br />
1<br />
3<br />
1<br />
<br />
2<br />
1<br />
6<br />
1<br />
3<br />
<br />
0 x<br />
<br />
2<br />
<br />
y<br />
<br />
3 <br />
1<br />
<br />
z<br />
3 <br />
1<br />
x<br />
<br />
2<br />
<br />
1<br />
y <br />
<br />
<br />
2<br />
<br />
z<br />
0<br />
<br />
<br />
1<br />
6<br />
1<br />
6<br />
2<br />
<br />
3<br />
1 <br />
<br />
3 X<br />
<br />
<br />
1<br />
<br />
<br />
<br />
<br />
Y<br />
3 <br />
<br />
1 <br />
Z<br />
<br />
3 <br />
<br />
a. Assume that you have a tetrahedral ML 4 molecule in which M is located at the origin of both<br />
coordinate systems. If the L‐atoms are found at the positions [½, ½, ½], [½,½, ½], [½,½,½],
and [½, ½, ½] in the coordinate system with fourfold quantization, what are their positions in<br />
the coordinate system with threefold quantization<br />
b. Use the real d‐orbital functions to find basis sets for the t 2 and e representations of the T d point<br />
group in the coordinate system with trigonal quantization.<br />
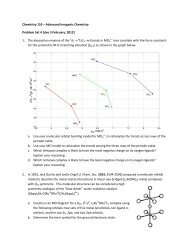
3. In 1993, Wilkinson and Hursthouse reported<br />
(Polyhedron, 1993, 12, 2009‐2012) the preparation<br />
and X‐ray crystal structure of Ir(O)(mes)3 (mes =<br />
mesityl, 2,4,6‐trimethylphenyl). The structure of the<br />
complex is shown to the right.<br />
a. Construct an MO diagram for C 3v Ir(O)(mes) 3<br />
using the following orbitals: five metal 5d orbitals, one set of three ligand σ orbitals, and the oxo<br />
2pσ + two 2pπ orbitals.<br />
b. Extend your diagram to illustrate the correlation between the trigonally quantized ML 4 orbitals<br />
and the MOs of Ir(O)(mes) 3 .<br />
c. Is there a unique set of d‐orbitals in C 3v symmetry that forms a basis for the e irreducible<br />
representation If so, what is that set of d‐orbitals If not, what is the implication for the<br />
molecular orbitals of e symmetry in Ir(O)(mes) 3 <br />
d. Using your MO diagram, fill in the d electrons and predict the ground‐state electronic<br />
configuration. With this prediction, is the metal‐oxo bond order shown above correct Why or<br />
why not Do you think that a complex of the form [Ir(O)(mes) 3 ] 2− could exist Why or why not<br />
e. Does Ir(O)(mes) 3 represent a breach of the oxo wall Why or why not