Chemistry 153 â Advanced Inorganic Chemistry Problem Set 5 (due ...
Chemistry 153 â Advanced Inorganic Chemistry Problem Set 5 (due ...
Chemistry 153 â Advanced Inorganic Chemistry Problem Set 5 (due ...
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
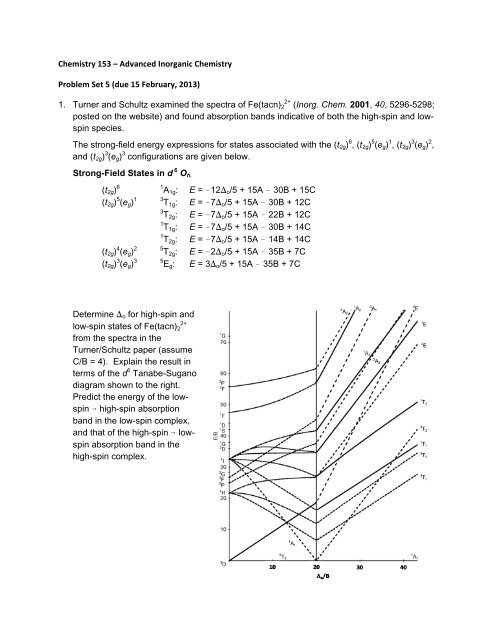
<strong>Chemistry</strong> <strong>153</strong> – <strong>Advanced</strong> <strong>Inorganic</strong> <strong>Chemistry</strong><strong>Problem</strong> <strong>Set</strong> 5 (<strong>due</strong> 15 February, 2013)1. Turner and Schultz examined the spectra of Fe(tacn) 2 2+ (Inorg. Chem. 2001, 40, 5296-5298;posted on the website) and found absorption bands indicative of both the high-spin and lowspinspecies.The strong-field energy expressions for states associated with the (t 2g ) 6 , (t 2g ) 5 (e g ) 1 , (t 2g ) 3 (e g ) 2 ,and (t 2g ) 3 (e g ) 3 configurations are given below.Strong-Field States in d 6 O h.(t 2g ) 6 1 A 1g : E = !12) o /5 + 15A ! 30B + 15C(t 2g ) 5 (e g ) 1 3 T 1g : E = !7) o /5 + 15A ! 30B + 12C3 T 2g : E = !7) o /5 + 15A ! 22B + 12C1 T 1g : E = !7) o /5 + 15A ! 30B + 14C1 T 2g : E = !7) o /5 + 15A ! 14B + 14C(t 2g ) 4 (e g ) 2 5 T 2g : E = !2) o /5 + 15A ! 35B + 7C(t 2g ) 3 (e g ) 3 5 E g : E = 3) o /5 + 15A ! 35B + 7CDetermine ) o for high-spin andlow-spin states of Fe(tacn) 22+from the spectra in theTurner/Schultz paper (assumeC/B = 4). Explain the result interms of the d 6 Tanabe-Suganodiagram shown to the right.Predict the energy of the lowspin6 high-spin absorptionband in the low-spin complex,and that of the high-spin 6 lowspinabsorption band in thehigh-spin complex.
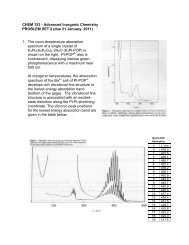
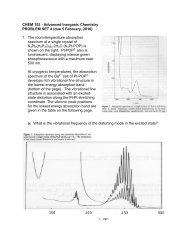
2. The room-temperature absorption spectrum of asingle crystal of K 4 Pt 2 (H 2 P 2 O 5 ) 4·2H 2 O (K 4 Pt-POP) is shown on the right. Pt-POP 4- also isluminescent, displaying intense greenphosphorescence with a maximum near 500nm.At cryogenic temperatures, the absorptionspectrum of the Ba 2+ salt of Pt-POP 4 developsrich vibrational fine structure in the lowestenergy absorption band (bottom of page). Thevibrational fine structure is associated with anexcited-state distortion along the Pt-Pt stretchingcoordinate. The vibronic peak positions for thelowest energy absorption band are given in thetable below.v , nm0 476.01 472.52 469.13 465.74 462.45 459.26 456.17 452.98 449.89 446.710 443.511 440.412 437.413 434.414 431.515 428.716 425.817 423.218 420.319 417.5
a. What is the vibrational frequency of the distorting mode in the excited state?b. What value of the Huang-Rhys parameter (SHR) gives the best fit to the lowest energyabsorption band?c. If the force constant for the Pt-Pt stretching mode is 1.0 mdyne/Å, what is the magnitudeof the distortion along the Pt-Pt coordinate in the excited state?The phosphorescence spectrum of crystalline Ba2Pt-POP at 10 K also displays rich finestructure in the Pt-Pt vibrational mode (bottom of the page). The vibronic peak positionsfor the phosphorescence band are given in the table below.d. What is the vibrational frequency of the distorting mode in the ground state?e. What value of the Huang-Rhys parameter (SHR) gives the best fit to thephosphorescence band?f. On the basis of your fit to the phosphorescence spectrum, what is the magnitude of thedistortion along the Pt-Pt coordinate in the excited state? How does this value compareto that extracted from the fit to the absorption spectrum?g. The Pt-Pt distance in the ground state of Pt-POP4! is 2.92 Å. On the basis of thestructured absorption and phosphorescence band profiles, what do you estimate for thePt-Pt distance in the excited state?h. Several time-resolved X-ray diffraction studies of Pt-POP 4 have appeared recently.Compare your estimated excited-state Pt-Pt bond distance to the results of one of thesestudies (include the reference of the study).Ba 2 POPPhosphorescencev , nm0 476.51 479.02 481.63 484.24 486.85 489.56 492.37 495.18 497.89 500.610 503.311 506.112 509.113 512.014 515.015 517.916 520.917 523.918 526.919 530.120 533.321 536.5