CHEM 153 - Advanced Inorganic Chemistry PROBLEM SET 4 (due ...
CHEM 153 - Advanced Inorganic Chemistry PROBLEM SET 4 (due ...
CHEM 153 - Advanced Inorganic Chemistry PROBLEM SET 4 (due ...
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
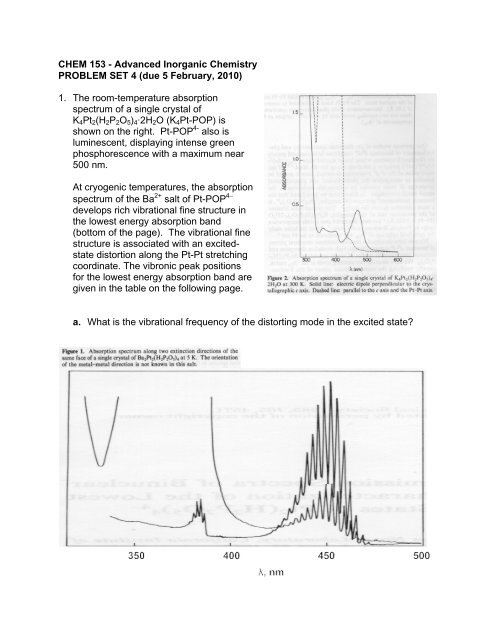
<strong>CHEM</strong> <strong>153</strong> - <strong>Advanced</strong> <strong>Inorganic</strong> <strong>Chemistry</strong><strong>PROBLEM</strong> <strong>SET</strong> 4 (<strong>due</strong> 5 February, 2010)1. The room-temperature absorptionspectrum of a single crystal ofK 4 Pt 2 (H 2 P 2 O 5 ) 4·2H 2 O (K 4 Pt-POP) isshown on the right. Pt-POP 4- also isluminescent, displaying intense greenphosphorescence with a maximum near500 nm.At cryogenic temperatures, the absorptionspectrum of the Ba 2+ salt of Pt-POP 4develops rich vibrational fine structure inthe lowest energy absorption band(bottom of the page). The vibrational finestructure is associated with an excitedstatedistortion along the Pt-Pt stretchingcoordinate. The vibronic peak positionsfor the lowest energy absorption band aregiven in the table on the following page.a. What is the vibrational frequency of the distorting mode in the excited state?
. What value of the Huang-Rhys parameter (SHR) gives the best fit tothe lowest energy absorption band?Ba 2 Pt-POPAbsorptionc. If the force constant for the Pt-Pt stretching mode is 1.0 mdyne/Å,what is the magnitude of the distortion along the Pt-Pt coordinate inthe excited state?The phosphorescence spectrum of crystalline Ba2Pt-POP at 10 K alsodisplays rich fine structure in the Pt-Pt vibrational mode (bottom of thepage). The vibronic peak positions for the phosphorescence band aregiven in the table on the following page.d. What is the vibrational frequency of the distorting mode in theground state?e. What value of the Huang-Rhys parameter (SHR) gives the best fit tothe phosphorescence band?v , nm0 476.01 472.52 469.13 465.74 462.45 459.26 456.17 452.98 449.89 446.710 443.511 440.412 437.413 434.414 431.515 428.716 425.817 423.218 420.319 417.5f. On the basis of your fit to the phosphorescence spectrum, what isthe magnitude of the distortion along the Pt-Pt coordinate in theexcited state? How does this value compare to that extracted fromthe fit to the absorption spectrum?g. The Pt-Pt distance in the ground state of Pt-POP4! is 2.92 Å. On the basis of thestructured absorption and phosphorescence band profiles, what do you estimatefor the Pt-Pt distance in the excited state?Ba 2 POPPhosphorescencev , nm0 476.51 479.02 481.63 484.24 486.85 489.56 492.37 495.18 497.89 500.610 503.311 506.112 509.113 512.014 515.015 517.916 520.917 523.918 526.919 530.120 533.321 536.5
2. Consider a binuclear metal complex constructed fromtwo square-planar ML 4 fragments where the L ligands areσ-donors. There are two limiting conformations in theresulting M 2 L 8 complex: in one the ligands are eclipsed andin the other they are staggered.a. Draw the two conformations of the binuclear metalcomplex and assign each to a symmetry pointgroup.b. Construct an MO diagram for each conformation using the following orbitals: fiveM d orbitals and four L σ orbitals. Assume that M-L σ-bonding is quite strong.c. Assume that ML 4 has a d 4 electron configuration, and that each ligand Lcontributes two σ electrons.1. Predict the preferred ground-state conformation of the corresponding M 2 L 8complex, and give the electronic configuration and term symbol for the groundelectronic state. Determine the metal-metal bond order.2. Identify the spin-allowed electronic transitions involving the d-orbitals,determine the term symbols for the excited states, and predict the relativeenergy ordering of these states.3. Identify the electric-dipole-allowed transitions and determine the polarizationof light that will induce the transition(s).d. Assume that ML 4 has a d 6 electron configuration, and that each ligand Lcontributes two σ electrons.1. Predict the preferred ground-state conformation of the corresponding M 2 L 8complex, and give the electronic configuration and term symbol for the groundelectronic state. Determine the metal-metal bond order.2. Identify the spin-allowed electronic transitions involving the d-orbitals,determine the term symbols for the excited states, and predict the relativeenergy ordering of these states.3. Identify the electric-dipole-allowed transitions and determine the polarizationof light that will induce the transition(s).
e. Assume that ML 4 has a d 7 electron configuration, and that each ligand Lcontributes two σ electrons.1. Predict the preferred ground-state conformation of the corresponding M 2 L 8complex, and give the electronic configuration and term symbol for the groundelectronic state. Determine the metal-metal bond order.2. Identify the spin-allowed electronic transitions involving the d-orbitals,determine the term symbols for the excited states, and predict the relativeenergy ordering of these states.3. Identify the electric-dipole-allowed transitions and determine the polarizationof light that will induce the transition(s).4