CHEM 153 - Advanced Inorganic Chemistry PROBLEM SET 2 (due ...
CHEM 153 - Advanced Inorganic Chemistry PROBLEM SET 2 (due ...
CHEM 153 - Advanced Inorganic Chemistry PROBLEM SET 2 (due ...
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
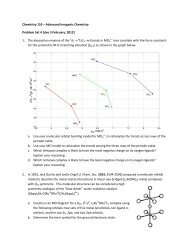
<strong>CHEM</strong> <strong>153</strong> - <strong>Advanced</strong> <strong>Inorganic</strong> <strong>Chemistry</strong><strong>PROBLEM</strong> <strong>SET</strong> 2 (<strong>due</strong> 25 January, 2013)1. Consider the following oxo complexes:Part 1:Part 2:Part 3:a. [L 5 VO] 2+ V IV d 1b. [L 5 CrO] 3+ Cr V d 1c. [L 5 CrO] 2+ Cr IV d 2d. [L 5 MnO] 3+ Mn V d 2e. [L 5 MnO] 2+ Mn IV d 3f. [L 5 FeO] 2+ Fe IV d 4Construct a ligand-field (LF) diagram for C 4v [L 5 MO] n+ (L is an uncharged ligand, for example, H 2 Oor NH 3 ) using the following orbitals: five metal 3d orbitals, one set of five ligand orbitals, and theoxo 2p + two 2p orbitals.Using your LF diagram, for each of the above examples, fill in the d electrons and predict theground-state electronic configuration. With this prediction, calculate the metal-oxo bond order.Based on your results, do you think that a complex of the form [L 5 CoO] n+ could exist? Why orwhy not?a. Larry Que and coworkers reported the synthesis and isolation of a nonheme ferryl complex(Science 2003, 299, 1037-1039). What are the Fe-O bond properties (bond length and bondorder)? Are they consistent with your predictions for 6-coordinate Fe IV ? How do the reportedMössbauer spectra support these findings?b. The electronic structures and spectroscopic properties of Que’s nonheme ferryl complexhave been investigated in Ed Solomon’s laboratory (J. Am. Chem. Soc. 2004, 126, 5378-7379). An absorption system in the 10,000 cm 1 region, called band I by Solomon, wasassigned to the (xy)(x 2 y 2 ) transition in the ferryl spectrum. What is the electronicconfiguration of the excited state formed by this transition? What is the value of S (the spinquantum number) for this excited state? The energies of the transitions associated withbands I and II are at much lower energies than the corresponding transitions in d 1 metal-oxocomplexes such as [L 5 VO] 2+ and [L 5 CrO] 3+ . How does Solomon account for thesedifferences?
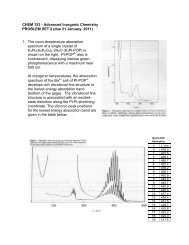
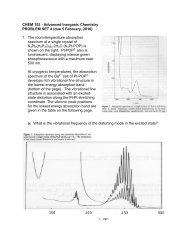
3. The 5 K luminescence spectrum of trans-[Re(O) 2 (pyridine) 4 ]I is shown below, along with a table ofpeak positions. The luminescence lifetime at 5 K is about 7 s.a. Construct a ligand-field (LF) diagram for D 4h trans-[Re(O) 2 (L) 4 ] + (L is anuncharged donor ligand ) using the following orbitals: five metal 3d orbitals,one set of four ligand orbitals, and a 2p + two 2p orbitals for each oxoligand.b. Determine the frequencies and suggest assignments for the vibrationalmode(s) giving rise to the fine structure in the spectrum.c. Perform a Franck-Condon analysis of the vibrational fine structure in theluminescence profile and determine what S HR -value(s) give the best fit to theobserved spectrum.d. Rationalize the vibronic structure in this band on the basis of an assignment forthe electronic transition giving rise to the luminescence.PeakPosition(cm 1 )16 78416 57316 36415 87715 66615 46014 97214 76414 56114 07313 86613 66813 17812 97412 78312 28612 09111 91111 40011 21111 028