Section 1.1 Section 1.2 Section 1.3 - The Student Room
Section 1.1 Section 1.2 Section 1.3 - The Student Room
Section 1.1 Section 1.2 Section 1.3 - The Student Room
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
SECTION 4<br />
<strong>Section</strong> 4.6<br />
1 a i Li(s) + Cl 2<br />
(g) Æ LiCl(s)<br />
ii Li(s) Æ Li(g)<br />
iii Cl 2<br />
(g) Æ Cl(g)<br />
iv Li(g) Æ Li + (g) + e –<br />
v Cl(g) + e – Æ Cl – (g)<br />
vi Li + (g) + Cl – (g) Æ LiCl(s)<br />
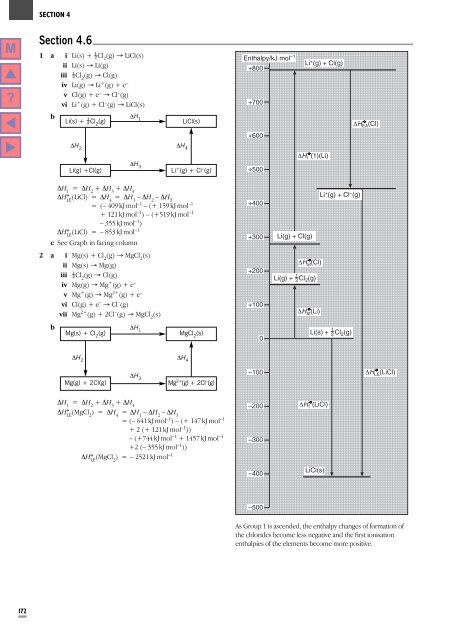
Enthalpy/kJ mol –1<br />
+800<br />
+700<br />
Li + (g) + Cl(g)<br />
b<br />
Li(s) + Cl 2<br />
(g)<br />
DH 1<br />
LiCl(s)<br />
DH EA (Cl)<br />
+600<br />
DH 2<br />
DH 4<br />
Li(g) +Cl(g)<br />
DH 3<br />
Li + (g) + Cl – (g)<br />
+500<br />
DH i (1)(Li)<br />
DH 1<br />
= DH 2<br />
+ DH 3<br />
+ DH 4<br />
DH! LE<br />
(LiCl) = DH 4<br />
= DH 1<br />
– DH 2<br />
– DH 3<br />
= (– 409 kJ mol –1 – (+ 159 kJ mol –1<br />
+ 121 kJ mol –1 ) – (+519 kJ mol –1<br />
– 355 kJ mol –1 )<br />
DH! LE<br />
(LiCl) = – 853 kJ mol –1<br />
<br />
c See Graph in facing column<br />
2 a i Mg(s) + Cl 2<br />
(g) Æ MgCl 2<br />
(s)<br />
ii Mg(s) Æ Mg(g)<br />
iii Cl 2<br />
(g) Æ Cl(g)<br />
iv Mg(g) Æ Mg + (g) + e –<br />
v Mg + (g) Æ Mg 2+ (g) + e –<br />
vi Cl(g) + e – Æ Cl – (g)<br />
vii Mg 2+ (g) + 2Cl – (g) Æ MgCl 2<br />
(s)<br />
b<br />
Mg(s) + Cl 2<br />
(g)<br />
DH 1<br />
MgCl 2<br />
(s)<br />
+400<br />
+300<br />
+200<br />
+100<br />
0<br />
Li(g) + Cl(g)<br />
DH at (Cl)<br />
1<br />
Li(g) + 2 Cl 2 (g)<br />
DH at (Li)<br />
Li + (g) + Cl – (g)<br />
1<br />
Li(s) + 2 Cl 2 (g)<br />
DH 2<br />
DH 4<br />
Mg(g) + 2Cl(g)<br />
DH 3<br />
Mg 2+ (g) + 2Cl – (g)<br />
–100<br />
DH LE (LiCl)<br />
DH 1<br />
= DH 2<br />
+ DH 3<br />
+ DH 4<br />
DH! LE<br />
(MgCl 2<br />
) = DH 4<br />
= DH 1<br />
– DH 2<br />
– DH 3<br />
= (– 641 kJ mol –1 ) – (+ 147 kJ mol –1<br />
+ 2 (+ 121 kJ mol –1 ))<br />
– (+744 kJ mol –1 + 1457 kJ mol –1<br />
+2 (– 355 kJ mol –1 ))<br />
DH! LE<br />
(MgCl 2<br />
) = – 2521 kJ mol –1<br />
–200 DH f (LiCl)<br />
–300<br />
–400<br />
LiCl(s)<br />
–500<br />
As Group 1 is ascended, the enthalpy changes of formation of<br />
the chlorides become less negative and the first ionisation<br />
enthalpies of the elements become more positive.<br />
172















![ISI Web of Knowledge [v.4.10] - All Databases Results - Benjamin-Mills](https://img.yumpu.com/39253071/1/184x260/isi-web-of-knowledge-v410-all-databases-results-benjamin-mills.jpg?quality=85)