Section 1.1 Section 1.2 Section 1.3 - The Student Room
Section 1.1 Section 1.2 Section 1.3 - The Student Room
Section 1.1 Section 1.2 Section 1.3 - The Student Room
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
SECTION 13<br />
2 a, b 1 mole glycerol (propane-1,2,3-triol)<br />
2 moles oleic acid<br />
1 mole linoleic acid<br />
3 a Saturated fats are esters of fatty acids with no (or few) carbon double bonds.<br />
b Monounsaturated fats contain fatty acids with one carbon double bond (such as oleic acid).<br />
c Polyunsaturated fats contain a high proportion of fatty acid groups with two<br />
or more carbon double bonds (such as linoleic acid).<br />
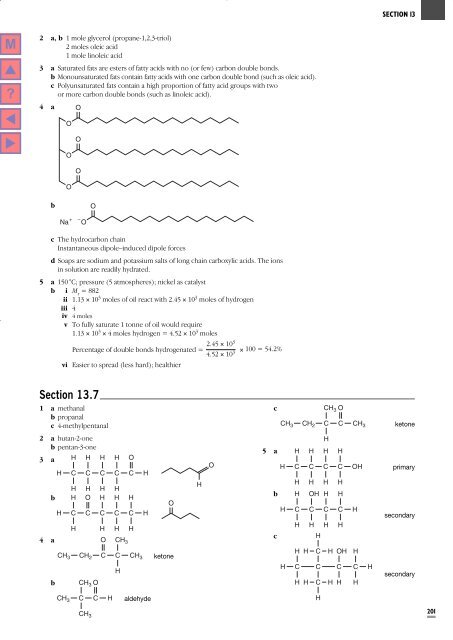
4 a O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
b<br />
O<br />
Na +<br />
– O<br />
c <strong>The</strong> hydrocarbon chain<br />
Instantaneous dipole–induced dipole forces<br />
d Soaps are sodium and potassium salts of long chain carboxylic acids. <strong>The</strong> ions<br />
in solution are readily hydrated.<br />
5 a 150 °C; pressure (5 atmospheres); nickel as catalyst<br />
b i M r<br />
= 882<br />
ii <strong>1.1</strong>3 ¥ 10 3 moles of oil react with 2.45 ¥ 10 3 moles of hydrogen<br />
iii 4<br />
iv 4 moles<br />
v To fully saturate 1 tonne of oil would require<br />
<strong>1.1</strong>3 ¥ 10 3 ¥ 4 moles hydrogen = 4.52 ¥ 10 3 moles<br />
2.45 ¥ 10 3<br />
Percentage of double bonds hydrogenated = ¥ 100 = 54.2%<br />
vi Easier to spread (less hard); healthier<br />
primary<br />
CH 3<br />
<strong>Section</strong> 13.7<br />
1 a methanal<br />
c<br />
CH 3 O<br />
b propanal<br />
C<br />
c 4-methylpentanal<br />
CH 3 CH 2 C CH 3<br />
CH 3 C C H aldehyde<br />
H<br />
2 a butan-2-one<br />
H<br />
3<br />
b pentan-3-one<br />
5 a H H H H<br />
a H H H H O<br />
O<br />
H C C C C OH<br />
H C C C C C H<br />
4.52 ¥ 10 3<br />
H<br />
H H H H<br />
H H H H<br />
b H OH H H<br />
b H O H H H<br />
O<br />
H C C C C H<br />
H C C C C C H<br />
H H H H<br />
H H H H<br />
4 a<br />
O CH 3<br />
c<br />
H<br />
CH 3 CH 2 C C CH 3 ketone<br />
H H C H OH H<br />
H<br />
H C C C C H<br />
b CH 3 O<br />
H H C H H H<br />
ketone<br />
secondary<br />
secondary<br />
201















![ISI Web of Knowledge [v.4.10] - All Databases Results - Benjamin-Mills](https://img.yumpu.com/39253071/1/184x260/isi-web-of-knowledge-v410-all-databases-results-benjamin-mills.jpg?quality=85)