Section 1.1 Section 1.2 Section 1.3 - The Student Room
Section 1.1 Section 1.2 Section 1.3 - The Student Room
Section 1.1 Section 1.2 Section 1.3 - The Student Room
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
SECTION 14<br />
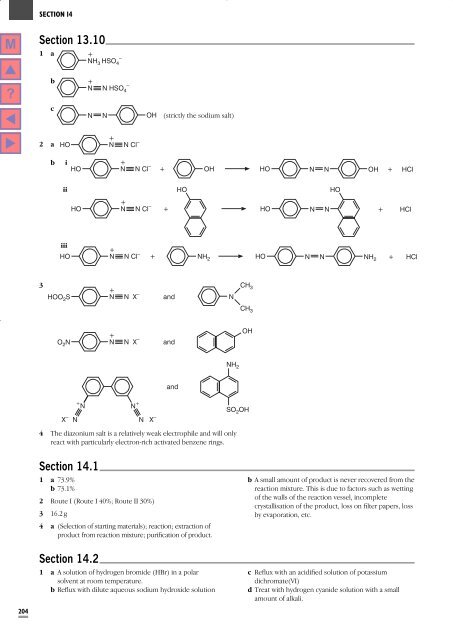
<strong>Section</strong> 13.10<br />
1 a<br />
+<br />
–<br />
NH 3 HSO 4<br />
b<br />
+<br />
–<br />
N N HSO 4<br />
c<br />
N N OH<br />
(strictly the sodium salt)<br />
2 a<br />
HO<br />
+<br />
N N Cl –<br />
b<br />
i<br />
+<br />
HO N N Cl – + OH HO N N OH + HCl<br />
ii<br />
HO<br />
+<br />
HO N N Cl – + HO N N<br />
+ HCl<br />
HO<br />
iii<br />
+<br />
HO N N Cl – + NH 2 HO N N NH 2 + HCl<br />
3<br />
HOO 2 S<br />
+<br />
N N X –<br />
and<br />
N<br />
CH 3<br />
CH 3<br />
O 2 N<br />
+<br />
N N X –<br />
and<br />
OH<br />
NH 2<br />
and<br />
X – N<br />
+ N<br />
N + N X –<br />
4 <strong>The</strong> diazonium salt is a relatively weak electrophile and will only<br />
react with particularly electron-rich activated benzene rings.<br />
SO 2 OH<br />
204<br />
<strong>Section</strong> 14.1<br />
1 a 73.9%<br />
b 73.1%<br />
2 Route I (Route I 40%; Route II 30%)<br />
3 16.2 g<br />
4 a (Selection of starting materials); reaction; extraction of<br />
product from reaction mixture; purification of product.<br />
<strong>Section</strong> 14.2<br />
1 a A solution of hydrogen bromide (HBr) in a polar<br />
solvent at room temperature.<br />
b Reflux with dilute aqueous sodium hydroxide solution<br />
b A small amount of product is never recovered from the<br />
reaction mixture. This is due to factors such as wetting<br />
of the walls of the reaction vessel, incomplete<br />
crystallisation of the product, loss on filter papers, loss<br />
by evaporation, etc.<br />
c Reflux with an acidified solution of potassium<br />
dichromate(VI)<br />
d Treat with hydrogen cyanide solution with a small<br />
amount of alkali.















![ISI Web of Knowledge [v.4.10] - All Databases Results - Benjamin-Mills](https://img.yumpu.com/39253071/1/184x260/isi-web-of-knowledge-v410-all-databases-results-benjamin-mills.jpg?quality=85)