Section 1.1 Section 1.2 Section 1.3 - The Student Room
Section 1.1 Section 1.2 Section 1.3 - The Student Room
Section 1.1 Section 1.2 Section 1.3 - The Student Room
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
SECTION 13<br />
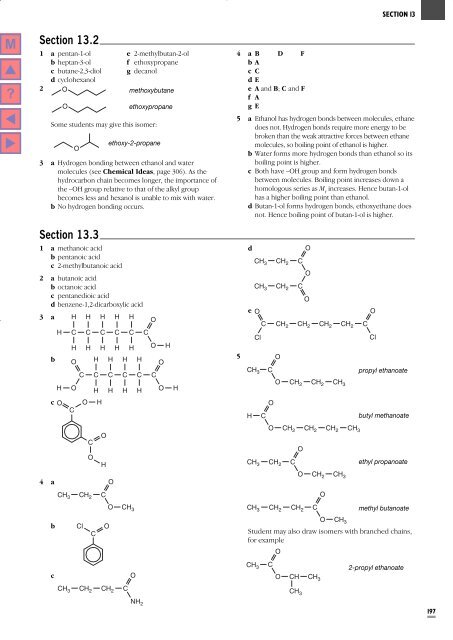
<strong>Section</strong> 13.2<br />
1 a pentan-1-ol e 2-methylbutan-2-ol<br />
b heptan-3-ol<br />
f ethoxypropane<br />
c butane-2,3-diol g decanol<br />
d cyclohexanol<br />
2 O<br />
methoxybutane<br />
O<br />
ethoxypropane<br />
Some students may give this isomer:<br />
O<br />
ethoxy-2-propane<br />
3 a Hydrogen bonding between ethanol and water<br />
molecules (see Chemical Ideas, page 306). As the<br />
hydrocarbon chain becomes longer, the importance of<br />
the –OH group relative to that of the alkyl group<br />
becomes less and hexanol is unable to mix with water.<br />
b No hydrogen bonding occurs.<br />
4 a B D F<br />
bA<br />
cC<br />
dE<br />
eAand B; C and F<br />
f A<br />
gE<br />
5 a Ethanol has hydrogen bonds between molecules, ethane<br />
does not. Hydrogen bonds require more energy to be<br />
broken than the weak attractive forces between ethane<br />
molecules, so boiling point of ethanol is higher.<br />
b Water forms more hydrogen bonds than ethanol so its<br />
boiling point is higher.<br />
c Both have –OH group and form hydrogen bonds<br />
between molecules. Boiling point increases down a<br />
homologous series as M r<br />
increases. Hence butan-1-ol<br />
has a higher boiling point than ethanol.<br />
d Butan-1-ol forms hydrogen bonds, ethoxyethane does<br />
not. Hence boiling point of butan-1-ol is higher.<br />
<strong>Section</strong> 13.3<br />
O<br />
CH 3<br />
CH 2<br />
1 a methanoic acid<br />
d<br />
b pentanoic acid<br />
CH<br />
c 2-methylbutanoic acid<br />
3 CH 2 C<br />
CH 3 CH 2 C<br />
CH 3<br />
2 a butanoic acid<br />
O<br />
b octanoic acid<br />
CH 3 CH 2 C<br />
c pentanedioic acid<br />
O<br />
d benzene-1,2-dicarboxylic acid<br />
e O<br />
O<br />
3 a H H H H H<br />
O<br />
C CH 2 CH 2 CH 2 CH 2 C<br />
H C C C C C C<br />
Cl<br />
Cl<br />
H H H H H O H<br />
b O H H H H<br />
O<br />
5<br />
O<br />
C C C C C C<br />
C<br />
propyl ethanoate<br />
H O<br />
O<br />
H H H H O H<br />
CH 2 CH 2 CH 3<br />
c O O H<br />
O<br />
C<br />
H C<br />
butyl methanoate<br />
O CH 2 CH 2 CH 2 CH 3<br />
O<br />
C<br />
O<br />
O<br />
H<br />
CH 3 CH 2 C<br />
ethyl propanoate<br />
O CH 2 CH 3<br />
O CH 3<br />
CH 3 CH 2 CH 2 C<br />
methyl butanoate<br />
4 a<br />
O<br />
CH 3 CH 2 C<br />
O<br />
O CH 3<br />
b Cl O<br />
C<br />
<strong>Student</strong> may also draw isomers with branched chains,<br />
for example<br />
O<br />
CH 3<br />
C<br />
2-propyl ethanoate<br />
c<br />
O<br />
O CH CH 3<br />
NH 2<br />
197















![ISI Web of Knowledge [v.4.10] - All Databases Results - Benjamin-Mills](https://img.yumpu.com/39253071/1/184x260/isi-web-of-knowledge-v410-all-databases-results-benjamin-mills.jpg?quality=85)