Section 1.1 Section 1.2 Section 1.3 - The Student Room
Section 1.1 Section 1.2 Section 1.3 - The Student Room
Section 1.1 Section 1.2 Section 1.3 - The Student Room
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
SECTION 14<br />
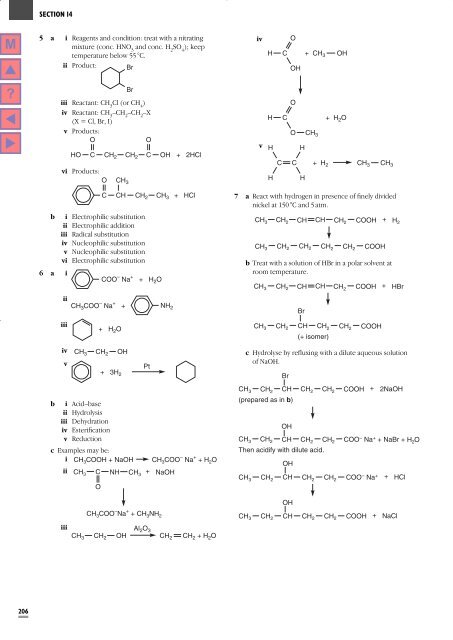
5 a i Reagents and condition: treat with a nitrating<br />
mixture (conc. HNO 3<br />
and conc. H 2<br />
SO 4<br />
); keep<br />
temperature below 55 °C.<br />
ii Product: Br<br />
iv<br />
H<br />
C<br />
O<br />
OH<br />
+ CH 3<br />
OH<br />
Br<br />
iii Reactant: CH 3<br />
Cl (or CH 4<br />
)<br />
iv Reactant: CH 3<br />
–CH 2<br />
–CH 2<br />
–X<br />
(X = Cl, Br, I)<br />
v Products:<br />
O<br />
O<br />
HO C<br />
C OH<br />
vi Products:<br />
O<br />
CH 2 CH 2<br />
+ HCl<br />
CH 3<br />
+ 2HCl<br />
v<br />
H<br />
H<br />
H<br />
O<br />
C<br />
+ H 2 O<br />
O CH 3<br />
H<br />
C C + H 2 CH 3 CH 3<br />
H<br />
b<br />
6 a i<br />
b<br />
C<br />
CH<br />
i Electrophilic substitution<br />
ii Electrophilic addition<br />
iii Radical substitution<br />
iv Nucleophilic substitution<br />
v Nucleophilic substitution<br />
vi Electrophilic substitution<br />
ii<br />
iii<br />
iv<br />
v<br />
COO – Na +<br />
CH 2 CH 3<br />
+ H 2 O<br />
CH 3 COO – Na + + NH 2<br />
+ H 2 O<br />
CH 3 CH 2 OH<br />
i Acid–base<br />
ii Hydrolysis<br />
iii Dehydration<br />
iv Esterification<br />
v Reduction<br />
+ 3H 2<br />
Pt<br />
c Examples may be:<br />
i CH 3 COOH + NaOH<br />
ii<br />
iii<br />
CH 3<br />
C NH CH 3<br />
O<br />
Al 2 O 3<br />
CH 3 CH 2 OH CH 2 CH 2 + H 2 O<br />
+<br />
CH 3 COO – Na + + CH 3 NH 2<br />
CH 3 COO – Na + + H 2 O<br />
NaOH<br />
7 a React with hydrogen in presence of finely divided<br />
nickel at 150 °C and 5 atm.<br />
CH 3 CH 2 CH CH CH 2 COOH + H 2<br />
CH 3 CH 2 CH 2 CH 2 CH 2 COOH<br />
b Treat with a solution of HBr in a polar solvent at<br />
room temperature.<br />
CH 3 CH 2 CH CH CH 2 COOH HBr<br />
Br<br />
CH 3 CH 2 CH CH 2<br />
(+ isomer)<br />
Br<br />
CH 2<br />
+<br />
CH 3 CH 2 CH CH 2 CH 2 COOH<br />
(prepared as in b)<br />
OH<br />
CH 3 CH 2 CH<br />
<strong>The</strong>n acidify with dilute acid.<br />
OH<br />
OH<br />
COOH<br />
c Hydrolyse by refluxing with a dilute aqueous solution<br />
of NaOH.<br />
2NaOH<br />
CH 2 CH COO – Na + 2<br />
+ NaBr + H 2 O<br />
CH 3 CH 2 CH CH 2 CH 2 COO – Na + + HCl<br />
CH3 CH 2 CH CH 2 CH 2 COOH + NaCl<br />
+<br />
206















![ISI Web of Knowledge [v.4.10] - All Databases Results - Benjamin-Mills](https://img.yumpu.com/39253071/1/184x260/isi-web-of-knowledge-v410-all-databases-results-benjamin-mills.jpg?quality=85)