Multiplet Effects in X-ray Absorption - Inorganic Chemistry and ...
Multiplet Effects in X-ray Absorption - Inorganic Chemistry and ...
Multiplet Effects in X-ray Absorption - Inorganic Chemistry and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
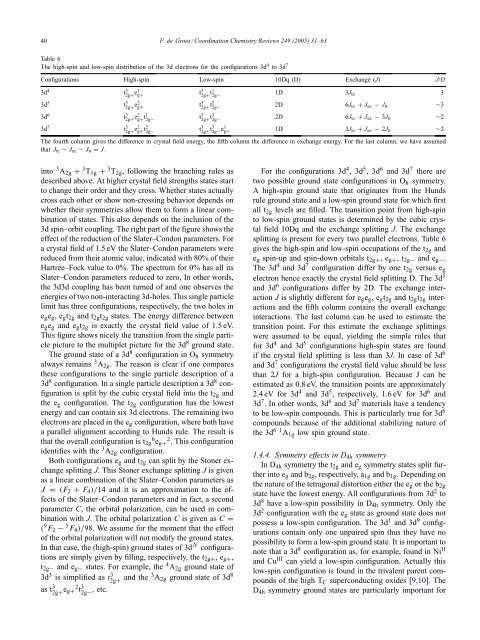
40 F. de Groot / Coord<strong>in</strong>ation <strong>Chemistry</strong> Reviews 249 (2005) 31–63Table 6The high-sp<strong>in</strong> <strong>and</strong> low-sp<strong>in</strong> distribution of the 3d electrons for the configurations 3d 4 to 3d 7Configurations High-sp<strong>in</strong> Low-sp<strong>in</strong> 10Dq (D) Exchange (J) J/D3d 4 t 3 2g+ e1 g+ t 3 2g+ t1 2g− 1D 3J te 33d 5 t 3 2g+ e2 g+ t 3 2g+ t2 2g− 2D 6J te + J ee − J tt ∼33d 6 t 3 2g+ e2 g+ t1 2g− t 3 2g+ t3 2g− 2D 6J te + J ee − 3J tt ∼23d 7 t 3 2g+ e2 g+ t2 2g− t 3 2g+ t3 2g− e1 g+ 1D 3J te + J ee − 2J tt ∼2The fourth column gives the difference <strong>in</strong> crystal field energy, the fifth column the difference <strong>in</strong> exchange energy. For the last column, we have assumedthat J te ∼ J ee ∼ J tt = J.<strong>in</strong>to 3 A 2g + 3 T 1g + 3 T 2g , follow<strong>in</strong>g the branch<strong>in</strong>g rules asdescribed above. At higher crystal field strengths states startto change their order <strong>and</strong> they cross. Whether states actuallycross each other or show non-cross<strong>in</strong>g behavior depends onwhether their symmetries allow them to form a l<strong>in</strong>ear comb<strong>in</strong>ationof states. This also depends on the <strong>in</strong>clusion of the3d sp<strong>in</strong>–orbit coupl<strong>in</strong>g. The right part of the figure shows theeffect of the reduction of the Slater–Condon parameters. Fora crystal field of 1.5 eV the Slater–Condon parameters werereduced from their atomic value, <strong>in</strong>dicated with 80% of theirHartree–Fock value to 0%. The spectrum for 0% has all itsSlater–Condon parameters reduced to zero, In other words,the 3d3d coupl<strong>in</strong>g has been turned of <strong>and</strong> one observes theenergies of two non-<strong>in</strong>teract<strong>in</strong>g 3d-holes. This s<strong>in</strong>gle particlelimit has three configurations, respectively, the two holes <strong>in</strong>e g e g ,e g t 2g <strong>and</strong> t 2g t 2g states. The energy difference betweene g e g <strong>and</strong> e g t 2g is exactly the crystal field value of 1.5 eV.This figure shows nicely the transition from the s<strong>in</strong>gle particlepicture to the multiplet picture for the 3d 8 ground state.The ground state of a 3d 8 configuration <strong>in</strong> O h symmetryalways rema<strong>in</strong>s 3 A 2g . The reason is clear if one comparesthese configurations to the s<strong>in</strong>gle particle description of a3d 8 configuration. In a s<strong>in</strong>gle particle description a 3d 8 configurationis split by the cubic crystal field <strong>in</strong>to the t 2g <strong>and</strong>the e g configuration. The t 2g configuration has the lowestenergy <strong>and</strong> can conta<strong>in</strong> six 3d electrons. The rema<strong>in</strong><strong>in</strong>g twoelectrons are placed <strong>in</strong> the e g configuration, where both havea parallel alignment accord<strong>in</strong>g to Hunds rule. The result isthat the overall configuration is t 2g 6 e g+ 2 . This configurationidentifies with the 3 A 2g configuration.Both configurations e g <strong>and</strong> t 2g can split by the Stoner exchangesplitt<strong>in</strong>g J. This Stoner exchange splitt<strong>in</strong>g J is givenas a l<strong>in</strong>ear comb<strong>in</strong>ation of the Slater–Condon parameters asJ = (F 2 + F 4 )/14 <strong>and</strong> it is an approximation to the effectsof the Slater–Condon parameters <strong>and</strong> <strong>in</strong> fact, a secondparameter C, the orbital polarization, can be used <strong>in</strong> comb<strong>in</strong>ationwith J. The orbital polarization C is given as C =( 9 F 2 − 5 F 4 )/98. We assume for the moment that the effectof the orbital polarization will not modify the ground states.In that case, the (high-sp<strong>in</strong>) ground states of 3d N configurationsare simply given by fill<strong>in</strong>g, respectively, the t 2g+ ,e g+ ,t 2g− <strong>and</strong> e g− states. For example, the 4 A 2g ground state of3d 3 is simplified as t 3 2g+ <strong>and</strong> the 3 A 2g ground state of 3d 8as t 3 2g+ e g+ 2 t 3 2g− , etc.For the configurations 3d 4 ,3d 5 ,3d 6 <strong>and</strong> 3d 7 there aretwo possible ground state configurations <strong>in</strong> O h symmetry.A high-sp<strong>in</strong> ground state that orig<strong>in</strong>ates from the Hundsrule ground state <strong>and</strong> a low-sp<strong>in</strong> ground state for which firstall t 2g levels are filled. The transition po<strong>in</strong>t from high-sp<strong>in</strong>to low-sp<strong>in</strong> ground states is determ<strong>in</strong>ed by the cubic crystalfield 10Dq <strong>and</strong> the exchange splitt<strong>in</strong>g J. The exchangesplitt<strong>in</strong>g is present for every two parallel electrons. Table 6gives the high-sp<strong>in</strong> <strong>and</strong> low-sp<strong>in</strong> occupations of the t 2g <strong>and</strong>e g sp<strong>in</strong>-up <strong>and</strong> sp<strong>in</strong>-down orbitals t 2g+ ,e g+ ,t 2g− <strong>and</strong> e g− .The 3d 4 <strong>and</strong> 3d 7 configuration differ by one t 2g versus e gelectron hence exactly the crystal field splitt<strong>in</strong>g D. The 3d 5<strong>and</strong> 3d 6 configurations differ by 2D. The exchange <strong>in</strong>teractionJ is slightly different for e g e g ,e g t 2g <strong>and</strong> t 2g t 2g <strong>in</strong>teractions<strong>and</strong> the fifth column conta<strong>in</strong>s the overall exchange<strong>in</strong>teractions. The last column can be used to estimate thetransition po<strong>in</strong>t. For this estimate the exchange splitt<strong>in</strong>gswere assumed to be equal, yield<strong>in</strong>g the simple rules thatfor 3d 4 <strong>and</strong> 3d 5 configurations high-sp<strong>in</strong> states are foundif the crystal field splitt<strong>in</strong>g is less than 3J. In case of 3d 6<strong>and</strong> 3d 7 configurations the crystal field value should be lessthan 2J for a high-sp<strong>in</strong> configuration. Because J can beestimated as 0.8 eV, the transition po<strong>in</strong>ts are approximately2.4 eV for 3d 4 <strong>and</strong> 3d 5 , respectively, 1.6 eV for 3d 6 <strong>and</strong>3d 7 . In other words, 3d 6 <strong>and</strong> 3d 7 materials have a tendencyto be low-sp<strong>in</strong> compounds. This is particularly true for 3d 6compounds because of the additional stabiliz<strong>in</strong>g nature ofthe 3d 61 A 1g low sp<strong>in</strong> ground state.1.4.4. Symmetry effects <strong>in</strong> D 4h symmetryIn D 4h symmetry the t 2g <strong>and</strong> e g symmetry states split further<strong>in</strong>to e g <strong>and</strong> b 2g , respectively, a 1g <strong>and</strong> b 1g . Depend<strong>in</strong>g onthe nature of the tetragonal distortion either the e g or the b 2gstate have the lowest energy. All configurations from 3d 2 to3d 8 have a low-sp<strong>in</strong> possibility <strong>in</strong> D 4h symmetry. Only the3d 2 configuration with the e g state as ground state does notpossess a low-sp<strong>in</strong> configuration. The 3d 1 <strong>and</strong> 3d 9 configurationsconta<strong>in</strong> only one unpaired sp<strong>in</strong> thus they have nopossibility to form a low-sp<strong>in</strong> ground state. It is important tonote that a 3d 8 configuration as, for example, found <strong>in</strong> Ni II<strong>and</strong> Cu III can yield a low-sp<strong>in</strong> configuration. Actually thislow-sp<strong>in</strong> configuration is found <strong>in</strong> the trivalent parent compoundsof the high T C superconduct<strong>in</strong>g oxides [9,10]. TheD 4h symmetry ground states are particularly important for