Multiplet Effects in X-ray Absorption - Inorganic Chemistry and ...
Multiplet Effects in X-ray Absorption - Inorganic Chemistry and ...
Multiplet Effects in X-ray Absorption - Inorganic Chemistry and ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
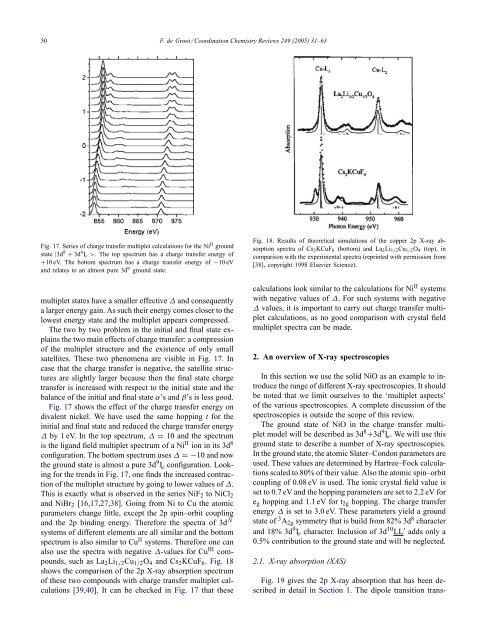
50 F. de Groot / Coord<strong>in</strong>ation <strong>Chemistry</strong> Reviews 249 (2005) 31–63Fig. 17. Series of charge transfer multiplet calculations for the Ni II groundstate |3d 8 + 3d 9 L¯ >. The top spectrum has a charge transfer energy of+10 eV. The bottom spectrum has a charge transfer energy of −10 eV<strong>and</strong> relates to an almost pure 3d 9 ground state.multiplet states have a smaller effective ∆ <strong>and</strong> consequentlya larger energy ga<strong>in</strong>. As such their energy comes closer to thelowest energy state <strong>and</strong> the multiplet appears compressed.The two by two problem <strong>in</strong> the <strong>in</strong>itial <strong>and</strong> f<strong>in</strong>al state expla<strong>in</strong>sthe two ma<strong>in</strong> effects of charge transfer: a compressionof the multiplet structure <strong>and</strong> the existence of only smallsatellites. These two phenomena are visible <strong>in</strong> Fig. 17. Incase that the charge transfer is negative, the satellite structuresare slightly larger because then the f<strong>in</strong>al state chargetransfer is <strong>in</strong>creased with respect to the <strong>in</strong>itial state <strong>and</strong> thebalance of the <strong>in</strong>itial <strong>and</strong> f<strong>in</strong>al state α’s <strong>and</strong> β’s is less good.Fig. 17 shows the effect of the charge transfer energy ondivalent nickel. We have used the same hopp<strong>in</strong>g t for the<strong>in</strong>itial <strong>and</strong> f<strong>in</strong>al state <strong>and</strong> reduced the charge transfer energy∆ by 1 eV. In the top spectrum, ∆ = 10 <strong>and</strong> the spectrumis the lig<strong>and</strong> field multiplet spectrum of a Ni II ion <strong>in</strong> its 3d 8configuration. The bottom spectrum uses ∆ =−10 <strong>and</strong> nowthe ground state is almost a pure 3d 9 L¯ configuration. Look<strong>in</strong>gfor the trends <strong>in</strong> Fig. 17, one f<strong>in</strong>ds the <strong>in</strong>creased contractionof the multiplet structure by go<strong>in</strong>g to lower values of ∆.This is exactly what is observed <strong>in</strong> the series NiF 2 to NiCl 2<strong>and</strong> NiBr 2 [16,17,27,38]. Go<strong>in</strong>g from Ni to Cu the atomicparameters change little, except the 2p sp<strong>in</strong>–orbit coupl<strong>in</strong>g<strong>and</strong> the 2p b<strong>in</strong>d<strong>in</strong>g energy. Therefore the spectra of 3d Nsystems of different elements are all similar <strong>and</strong> the bottomspectrum is also similar to Cu II systems. Therefore one canalso use the spectra with negative ∆-values for Cu III compounds,such as La 2 Li 1/2 Cu 1/2 O 4 <strong>and</strong> Cs 2 KCuF 6 . Fig. 18shows the comparison of the 2p X-<strong>ray</strong> absorption spectrumof these two compounds with charge transfer multiplet calculations[39,40]. It can be checked <strong>in</strong> Fig. 17 that theseFig. 18. Results of theoretical simulations of the copper 2p X-<strong>ray</strong> absorptionspectra of Cs 2 KCuF 6 (bottom) <strong>and</strong> La 2 Li 1/2 Cu 1/2 O 4 (top), <strong>in</strong>comparison with the experimental spectra (repr<strong>in</strong>ted with permission from[38], copyright 1998 Elsevier Science).calculations look similar to the calculations for Ni II systemswith negative values of ∆. For such systems with negative∆ values, it is important to carry out charge transfer multipletcalculations, as no good comparison with crystal fieldmultiplet spectra can be made.2. An overview of X-<strong>ray</strong> spectroscopiesIn this section we use the solid NiO as an example to <strong>in</strong>troducethe range of different X-<strong>ray</strong> spectroscopies. It shouldbe noted that we limit ourselves to the ‘multiplet aspects’of the various spectroscopies. A complete discussion of thespectroscopies is outside the scope of this review.The ground state of NiO <strong>in</strong> the charge transfer multipletmodel will be described as 3d 8 +3d 9 L¯. We will use thisground state to describe a number of X-<strong>ray</strong> spectroscopies.In the ground state, the atomic Slater–Condon parameters areused. These values are determ<strong>in</strong>ed by Hartree–Fock calculationsscaled to 80% of their value. Also the atomic sp<strong>in</strong>–orbitcoupl<strong>in</strong>g of 0.08 eV is used. The ionic crystal field value isset to 0.7 eV <strong>and</strong> the hopp<strong>in</strong>g parameters are set to 2.2 eV fore g hopp<strong>in</strong>g <strong>and</strong> 1.1 eV for t 2g hopp<strong>in</strong>g. The charge transferenergy ∆ is set to 3.0 eV. These parameters yield a groundstate of 3 A 2g symmetry that is build from 82% 3d 8 character<strong>and</strong> 18% 3d 9 L¯ character. Inclusion of 3d 10 LL ′ adds only a0.5% contribution to the ground state <strong>and</strong> will be neglected.2.1. X-<strong>ray</strong> absorption (XAS)Fig. 19 gives the 2p X-<strong>ray</strong> absorption that has been described<strong>in</strong> detail <strong>in</strong> Section 1. The dipole transition trans-