Multiplet Effects in X-ray Absorption - Inorganic Chemistry and ...
Multiplet Effects in X-ray Absorption - Inorganic Chemistry and ...
Multiplet Effects in X-ray Absorption - Inorganic Chemistry and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
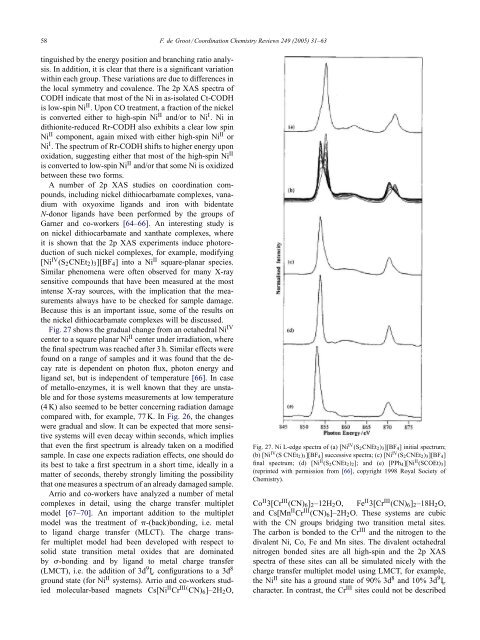
58 F. de Groot / Coord<strong>in</strong>ation <strong>Chemistry</strong> Reviews 249 (2005) 31–63t<strong>in</strong>guished by the energy position <strong>and</strong> branch<strong>in</strong>g ratio analysis.In addition, it is clear that there is a significant variationwith<strong>in</strong> each group. These variations are due to differences <strong>in</strong>the local symmetry <strong>and</strong> covalence. The 2p XAS spectra ofCODH <strong>in</strong>dicate that most of the Ni <strong>in</strong> as-isolated Ct-CODHis low-sp<strong>in</strong> Ni II . Upon CO treatment, a fraction of the nickelis converted either to high-sp<strong>in</strong> Ni II <strong>and</strong>/or to Ni I .Ni<strong>in</strong>dithionite-reduced Rr-CODH also exhibits a clear low sp<strong>in</strong>Ni II component, aga<strong>in</strong> mixed with either high-sp<strong>in</strong> Ni II orNi I . The spectrum of Rr-CODH shifts to higher energy uponoxidation, suggest<strong>in</strong>g either that most of the high-sp<strong>in</strong> Ni IIis converted to low-sp<strong>in</strong> Ni II <strong>and</strong>/or that some Ni is oxidizedbetween these two forms.A number of 2p XAS studies on coord<strong>in</strong>ation compounds,<strong>in</strong>clud<strong>in</strong>g nickel dithiocarbamate complexes, vanadiumwith oxyoxime lig<strong>and</strong>s <strong>and</strong> iron with bidentateN-donor lig<strong>and</strong>s have been performed by the groups ofGarner <strong>and</strong> co-workers [64–66]. An <strong>in</strong>terest<strong>in</strong>g study ison nickel dithiocarbamate <strong>and</strong> xanthate complexes, whereit is shown that the 2p XAS experiments <strong>in</strong>duce photoreductionof such nickel complexes, for example, modify<strong>in</strong>g[Ni IV (S 2 CNEt 2 ) 3 ][BF 4 ]<strong>in</strong>toaNi II square-planar species.Similar phenomena were often observed for many X-<strong>ray</strong>sensitive compounds that have been measured at the most<strong>in</strong>tense X-<strong>ray</strong> sources, with the implication that the measurementsalways have to be checked for sample damage.Because this is an important issue, some of the results onthe nickel dithiocarbamate complexes will be discussed.Fig. 27 shows the gradual change from an octahedral Ni IVcenter to a square planar Ni II center under irradiation, wherethe f<strong>in</strong>al spectrum was reached after 3 h. Similar effects werefound on a range of samples <strong>and</strong> it was found that the decayrate is dependent on photon flux, photon energy <strong>and</strong>lig<strong>and</strong> set, but is <strong>in</strong>dependent of temperature [66]. In caseof metallo-enzymes, it is well known that they are unstable<strong>and</strong> for those systems measurements at low temperature(4 K) also seemed to be better concern<strong>in</strong>g radiation damagecompared with, for example, 77 K. In Fig. 26, the changeswere gradual <strong>and</strong> slow. It can be expected that more sensitivesystems will even decay with<strong>in</strong> seconds, which impliesthat even the first spectrum is already taken on a modifiedsample. In case one expects radiation effects, one should doits best to take a first spectrum <strong>in</strong> a short time, ideally <strong>in</strong> amatter of seconds, thereby strongly limit<strong>in</strong>g the possibilitythat one measures a spectrum of an already damaged sample.Arrio <strong>and</strong> co-workers have analyzed a number of metalcomplexes <strong>in</strong> detail, us<strong>in</strong>g the charge transfer multipletmodel [67–70]. An important addition to the multipletmodel was the treatment of -(back)bond<strong>in</strong>g, i.e. metalto lig<strong>and</strong> charge transfer (MLCT). The charge transfermultiplet model had been developed with respect tosolid state transition metal oxides that are dom<strong>in</strong>atedby -bond<strong>in</strong>g <strong>and</strong> by lig<strong>and</strong> to metal charge transfer(LMCT), i.e. the addition of 3d 9 L¯ configurations to a 3d 8ground state (for Ni II systems). Arrio <strong>and</strong> co-workers studiedmolecular-based magnets Cs[Ni II Cr III( CN) 6 ]–2H 2 O,Fig. 27. Ni L-edge spectra of (a) [Ni IV (S 2 CNEt 2 ) 3 ][BF 4 ] <strong>in</strong>itial spectrum;(b) [Ni IV (S CNEt 2 ) 3 ][BF 4 ] successive spectra; (c) [Ni IV (S 2 CNEt 2 ) 3 ][BF 4 ]f<strong>in</strong>al spectrum; (d) [Ni II (S 2 CNEt 2 ) 2 ]; <strong>and</strong> (e) [PPh 4 ][Ni II (SCOEt) 3 ](repr<strong>in</strong>ted with permission from [66], copyright 1998 Royal Society of<strong>Chemistry</strong>).Co II 3[Cr III (CN) 6 ] 2 –12H 2 O, Fe II 3[Cr III (CN) 6 ] 2 –18H 2 O,<strong>and</strong> Cs[Mn II Cr III (CN) 6 ]–2H 2 O. These systems are cubicwith the CN groups bridg<strong>in</strong>g two transition metal sites.The carbon is bonded to the Cr III <strong>and</strong> the nitrogen to thedivalent Ni, Co, Fe <strong>and</strong> Mn sites. The divalent octahedralnitrogen bonded sites are all high-sp<strong>in</strong> <strong>and</strong> the 2p XASspectra of these sites can all be simulated nicely with thecharge transfer multiplet model us<strong>in</strong>g LMCT, for example,the Ni II site has a ground state of 90% 3d 8 <strong>and</strong> 10% 3d 9 L¯character. In contrast, the Cr III sites could not be described