42 F. de Groot / Coord<strong>in</strong>ation <strong>Chemistry</strong> Reviews 249 (2005) 31–63Table 8The matrix elements <strong>in</strong> SO 3 symmetry needed for the calculation of 2pX-<strong>ray</strong> absorption3d N → 2p 5 3d N +1 <strong>in</strong> SO 3 symmetryInitial state Transition F<strong>in</strong>al state〈0|0|0〉 〈0|1|1〉 〈0|0|0〉〈1|0|1〉 〈1|1|0〉 〈1|0|1〉〈1|1|1〉〈1|1|2〉〈2|0|2〉 〈2|1|1〉 〈2|0|2〉〈2|1|3〉〈3|0|3〉 〈3|1|2〉 〈3|0|3〉 ∗〈3|1|3〉〈3|1|4〉〈4|0|4〉 ∗ 〈4|1|3〉 ∗ 〈4|0|4〉 ∗〈4|1|4〉 ∗Boldface <strong>and</strong> ∗ matrix elements apply to, respectively, a 3d 0 <strong>and</strong>a3d 8configuration.low-sp<strong>in</strong> transition at 2.25 eV <strong>and</strong> also the important effectof the 3d sp<strong>in</strong>–orbit coupl<strong>in</strong>g. It can be observed that theatomic multiplet spectrum of Co II has a large number ofstates at low energy. All these states are part of the 4 F 9/2configuration that is split by the 3d sp<strong>in</strong>–orbit coupl<strong>in</strong>g. Afterapply<strong>in</strong>g a cubic crystal field, most of these multipletstates are shifted to higher energies <strong>and</strong> only four states rema<strong>in</strong>at low energy. These are the four states of 4 T 1g as<strong>in</strong>dicated <strong>in</strong> Table 7. These four states all rema<strong>in</strong> with<strong>in</strong>0.1 eV from the E 2 ground state. That this description isactually correct was shown <strong>in</strong> detail for the 2p X-<strong>ray</strong> absorptionspectrum of CoO [12], which has a cubic crystalfield of 1.2 eV. At 2.25 eV the high-sp<strong>in</strong> low-sp<strong>in</strong> transitionis evident. A new state is com<strong>in</strong>g from high energy<strong>and</strong> a G-symmetry state replaces the E 2 symmetrystate at the lowest energy. In fact there is a very <strong>in</strong>terest<strong>in</strong>gcomplication: due to the 3d sp<strong>in</strong>–orbit coupl<strong>in</strong>g theG-symmetry states of the 4 T 1g <strong>and</strong> 2 E g configurations mix<strong>and</strong> form l<strong>in</strong>ear comb<strong>in</strong>ations. Around the transition po<strong>in</strong>t,this l<strong>in</strong>ear comb<strong>in</strong>ation will have a sp<strong>in</strong>-state that is neitherhigh-sp<strong>in</strong> nor low-sp<strong>in</strong> <strong>and</strong> <strong>in</strong> fact a mixed sp<strong>in</strong>-state can befound.1.4.6. The effects on the X-<strong>ray</strong> absorption calculationsTable 8 gives all matrix element calculations that haveto be carried out for 3d N → 2p 5 3d N+1 transitions <strong>in</strong> SO 3symmetry for the J-values up to 4.We will use the transitions3d 0 → 2p 5 3d 1 as examples. 3d 0 conta<strong>in</strong>s only J = 0symmetry states, <strong>in</strong>dicated <strong>in</strong> boldface. This limits the calculationfor the ground state spectrum to only one groundstate, one transition <strong>and</strong> one f<strong>in</strong>al state matrix element, given<strong>in</strong> boldface. In case of 3d 8 Ni II the ground state has a 3 F 4configuration, <strong>in</strong>dicated as underl<strong>in</strong>ed. We are now go<strong>in</strong>g toapply the SO 3 → O h branch<strong>in</strong>g rule to this table. The J =4 ground state has transitions to J = 3 <strong>and</strong> 4 f<strong>in</strong>al states(Table 8).Table 9The matrix elements <strong>in</strong> O h symmetry needed for the calculation of 2pX-<strong>ray</strong> absorption3d N → 2p 5 3d N +1 <strong>in</strong> O h symmetryInitial state Transition F<strong>in</strong>al state〈A 1 |A 1 |A 1 〉 〈A 1 |T 1 |T 1 〉 〈A 1 |A 1 |A 1 〉〈T 1 |A 1 |T 1 〉 〈T 1 |T 1 |A 1 〉 〈T 1 |A 1 |T 1 〉 ∗〈T 1 |T 1 |T 1 〉〈T 1 |T 1 |E〉〈T 1 |T 1 |T 2 〉〈E|A 1 |E〉 〈E|T 1 |T 1 〉 〈E|A 1 |E〉 ∗〈E|T 1 |T 2 〉〈T 2 |A 1 |T 2 〉 ∗ 〈T 2 |T 1 |T 1 〉 ∗ 〈T 2 |A 1 |T 2 〉 ∗〈T 2 |T 1 |E〉 ∗〈T 2 |T 1 |T 2 〉 ∗〈T 2 |T 1 |A 2 〉 ∗〈A 2 |A 1 |A 2 〉 〈A 2 |T 1 |T 2 〉 〈A 2 |A 1 |A 2 〉 ∗Boldface <strong>and</strong> ∗ matrix elements apply to, respectively, a 3d 0 <strong>and</strong>a3d 8configuration.In octahedral symmetry one has to calculate five matricesfor the <strong>in</strong>itial <strong>and</strong> f<strong>in</strong>al states <strong>and</strong> thirteen transition matrices.Note that this is a general result for all even numbers of3d electrons, as there are only these five symmetries <strong>in</strong> O hsymmetry. In the 3d 0 case, the ground state branches to A 1<strong>and</strong> only three matrices are needed to generate the spectralshape: 〈A 1 |A 1 |A 1 〉 for the 3d 0 ground state, 〈A 1 |T 1 |T 1 〉 forthe dipole transition <strong>and</strong> 〈T 1 |A 1 |T 1 〉 for the 2p 5 3d 1 f<strong>in</strong>alstate (Table 9). The 3d 0 systems are rather special becausethey are not affected by ground state effects. Table 10 showsthat a 2p 5 3d 1 configuration has twelve representations <strong>in</strong>SO 3 symmetry that are branched to 25 representations <strong>in</strong> acubic field. From these 25 representations, only seven are of<strong>in</strong>terest for the calculation of the X-<strong>ray</strong> absorption spectralshape, because only these T 1 symmetry states obta<strong>in</strong> a f<strong>in</strong>ite<strong>in</strong>tensity.In the 3d 8 case, the ground state branches to T 2g , i.e.3 A 2g = T 1g ⊗A 2g = T 2g . The T 2g ground state yields dipoletransitions to four different f<strong>in</strong>al state symmetries, us<strong>in</strong>gT 2g ⊗ T 1u = T 1u + T 2u + E u + A 2u . Consequently the completespectral shape is given by calculat<strong>in</strong>g one ground stateTable 10The branch<strong>in</strong>g of the J-values <strong>in</strong> SO 3 symmetry to the representations <strong>in</strong>O h symmetry, us<strong>in</strong>g the degeneracies of the 2p 5 3d 1 f<strong>in</strong>al state <strong>in</strong> X-<strong>ray</strong>absorptionJ <strong>in</strong> SO 3 Degree Branch<strong>in</strong>gs Γ <strong>in</strong> O h Degree0 1 A 1u A 1u[0,4] 21 3 3 × T 1u A 2u[3] 32 4 4 × E u ,4× T 2u T 1u[1,3,4] 73 3 3 × A 2u ,3× T 1u ,3 × T 2u T 2u[2-4] 84 1 A 1u ,E u ,T 1u ,T 2u E u[2,4] 5∑12 25The symmetry <strong>in</strong> O h is given, <strong>in</strong>clud<strong>in</strong>g the SO 3 orig<strong>in</strong> of the states <strong>in</strong>square brackets.
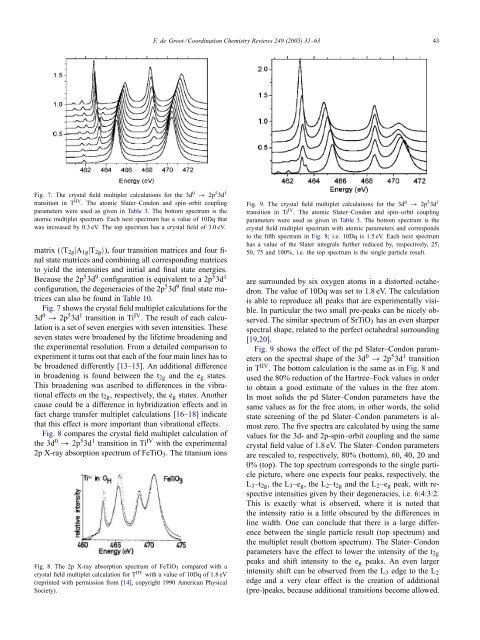
F. de Groot / Coord<strong>in</strong>ation <strong>Chemistry</strong> Reviews 249 (2005) 31–63 43Fig. 7. The crystal field multiplet calculations for the 3d 0 → 2p 5 3d 1transition <strong>in</strong> T IIV . The atomic Slater–Condon <strong>and</strong> sp<strong>in</strong>–orbit coupl<strong>in</strong>gparameters were used as given <strong>in</strong> Table 3. The bottom spectrum is theatomic multiplet spectrum. Each next spectrum has a value of 10Dq thatwas <strong>in</strong>creased by 0.3 eV. The top spectrum has a crystal field of 3.0 eV.matrix (〈T 2g |A 1g |T 2g 〉), four transition matrices <strong>and</strong> four f<strong>in</strong>alstate matrices <strong>and</strong> comb<strong>in</strong><strong>in</strong>g all correspond<strong>in</strong>g matricesto yield the <strong>in</strong>tensities <strong>and</strong> <strong>in</strong>itial <strong>and</strong> f<strong>in</strong>al state energies.Because the 2p 5 3d 9 configuration is equivalent to a 2p 5 3d 1configuration, the degeneracies of the 2p 5 3d 9 f<strong>in</strong>al state matricescan also be found <strong>in</strong> Table 10.Fig. 7 shows the crystal field multiplet calculations for the3d 0 → 2p 5 3d 1 transition <strong>in</strong> Ti IV . The result of each calculationis a set of seven energies with seven <strong>in</strong>tensities. Theseseven states were broadened by the lifetime broaden<strong>in</strong>g <strong>and</strong>the experimental resolution. From a detailed comparison toexperiment it turns out that each of the four ma<strong>in</strong> l<strong>in</strong>es has tobe broadened differently [13–15]. An additional difference<strong>in</strong> broaden<strong>in</strong>g is found between the t 2g <strong>and</strong> the e g states.This broaden<strong>in</strong>g was ascribed to differences <strong>in</strong> the vibrationaleffects on the t 2g , respectively, the e g states. Anothercause could be a difference <strong>in</strong> hybridization effects <strong>and</strong> <strong>in</strong>fact charge transfer multiplet calculations [16–18] <strong>in</strong>dicatethat this effect is more important than vibrational effects.Fig. 8 compares the crystal field multiplet calculation ofthe 3d 0 → 2p 5 3d 1 transition <strong>in</strong> Ti IV with the experimental2p X-<strong>ray</strong> absorption spectrum of FeTiO 3 . The titanium ionsFig. 8. The 2p X-<strong>ray</strong> absorption spectrum of FeTiO 3 compared with acrystal field multiplet calculation for T IIV with a value of 10Dq of 1.8 eV(repr<strong>in</strong>ted with permission from [14], copyright 1990 American PhysicalSociety).Fig. 9. The crystal field multiplet calculations for the 3d 0 → 2p 5 3d 1transition <strong>in</strong> Ti IV . The atomic Slater–Condon <strong>and</strong> sp<strong>in</strong>–orbit coupl<strong>in</strong>gparameters were used as given <strong>in</strong> Table 3. The bottom spectrum is thecrystal field multiplet spectrum with atomic parameters <strong>and</strong> correspondsto the fifth spectrum <strong>in</strong> Fig. 8; i.e. 10Dq is 1.5 eV. Each next spectrumhas a value of the Slater <strong>in</strong>tegrals further reduced by, respectively, 25,50, 75 <strong>and</strong> 100%, i.e. the top spectrum is the s<strong>in</strong>gle particle result.are surrounded by six oxygen atoms <strong>in</strong> a distorted octahedron.The value of 10Dq was set to 1.8 eV. The calculationis able to reproduce all peaks that are experimentally visible.In particular the two small pre-peaks can be nicely observed.The similar spectrum of SrTiO 3 has an even sharperspectral shape, related to the perfect octahedral surround<strong>in</strong>g[19,20].Fig. 9 shows the effect of the pd Slater–Condon parameterson the spectral shape of the 3d 0 → 2p 5 3d 1 transition<strong>in</strong> T IIV . The bottom calculation is the same as <strong>in</strong> Fig. 8 <strong>and</strong>used the 80% reduction of the Hartree–Fock values <strong>in</strong> orderto obta<strong>in</strong> a good estimate of the values <strong>in</strong> the free atom.In most solids the pd Slater–Condon parameters have thesame values as for the free atom, <strong>in</strong> other words, the solidstate screen<strong>in</strong>g of the pd Slater–Condon parameters is almostzero. The five spectra are calculated by us<strong>in</strong>g the samevalues for the 3d- <strong>and</strong> 2p-sp<strong>in</strong>–orbit coupl<strong>in</strong>g <strong>and</strong> the samecrystal field value of 1.8 eV. The Slater–Condon parametersare rescaled to, respectively, 80% (bottom), 60, 40, 20 <strong>and</strong>0% (top). The top spectrum corresponds to the s<strong>in</strong>gle particlepicture, where one expects four peaks, respectively, theL 3 –t 2g , the L 3 –e g , the L 2 –t 2g <strong>and</strong> the L 2 –e g peak, with respective<strong>in</strong>tensities given by their degeneracies, i.e. 6:4:3:2.This is exactly what is observed, where it is noted thatthe <strong>in</strong>tensity ratio is a little obscured by the differences <strong>in</strong>l<strong>in</strong>e width. One can conclude that there is a large differencebetween the s<strong>in</strong>gle particle result (top spectrum) <strong>and</strong>the multiplet result (bottom spectrum). The Slater–Condonparameters have the effect to lower the <strong>in</strong>tensity of the t 2gpeaks <strong>and</strong> shift <strong>in</strong>tensity to the e g peaks. An even larger<strong>in</strong>tensity shift can be observed from the L 3 edge to the L 2edge <strong>and</strong> a very clear effect is the creation of additional(pre-)peaks, because additional transitions become allowed.