Multiplet Effects in X-ray Absorption - Inorganic Chemistry and ...
Multiplet Effects in X-ray Absorption - Inorganic Chemistry and ...
Multiplet Effects in X-ray Absorption - Inorganic Chemistry and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
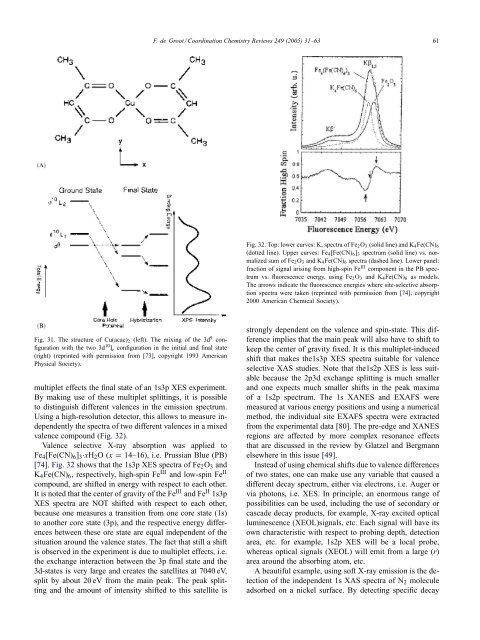
F. de Groot / Coord<strong>in</strong>ation <strong>Chemistry</strong> Reviews 249 (2005) 31–63 61Fig. 32. Top: lower curves: K, spectra of Fe 2 O 3 (solid l<strong>in</strong>e) <strong>and</strong> K 4 Fe(CN) 6(dotted l<strong>in</strong>e). Upper curves: Fe 4 [Fe(CN) 6 ] 3 spectrum (solid l<strong>in</strong>e) vs. normalizedsum of Fe 2 O 3 <strong>and</strong> K 4 Fe(CN) 6 spectra (dashed l<strong>in</strong>e). Lower panel:fraction of signal aris<strong>in</strong>g from high-sp<strong>in</strong> Fe III component <strong>in</strong> the PB spectrumvs. fluorescence energy, us<strong>in</strong>g Fe 2 O 3 <strong>and</strong> K 4 Fe(CN) 6 as models.The arrows <strong>in</strong>dicate the fluorescence energies where site-selective absorptionspectra were taken (repr<strong>in</strong>ted with permission from [74], copyright2000 American Chemical Society).Fig. 31. The structure of Cu(acac) 2 (left). The mix<strong>in</strong>g of the 3d 9 configurationwith the two 3d 10 L¯ configuration <strong>in</strong> the <strong>in</strong>itial <strong>and</strong> f<strong>in</strong>al state(right) (repr<strong>in</strong>ted with permission from [73], copyright 1993 AmericanPhysical Society).multiplet effects the f<strong>in</strong>al state of an 1s3p XES experiment.By mak<strong>in</strong>g use of these multiplet splitt<strong>in</strong>gs, it is possibleto dist<strong>in</strong>guish different valences <strong>in</strong> the emission spectrum.Us<strong>in</strong>g a high-resolution detector, this allows to measure <strong>in</strong>dependentlythe spectra of two different valences <strong>in</strong> a mixedvalence compound (Fig. 32).Valence selective X-<strong>ray</strong> absorption was applied toFe 4 [Fe(CN) 6 ] 3·xH 2 O(x = 14–16), i.e. Prussian Blue (PB)[74]. Fig. 32 shows that the 1s3p XES spectra of Fe 2 O 3 <strong>and</strong>K 4 Fe(CN) 6 , respectively, high-sp<strong>in</strong> Fe III <strong>and</strong> low-sp<strong>in</strong> Fe IIcompound, are shifted <strong>in</strong> energy with respect to each other.It is noted that the center of gravity of the Fe III <strong>and</strong> Fe II 1s3pXES spectra are NOT shifted with respect to each other,because one measures a transition from one core state (1s)to another core state (3p), <strong>and</strong> the respective energy differencesbetween these ore state are equal <strong>in</strong>dependent of thesituation around the valence states. The fact that still a shiftis observed <strong>in</strong> the experiment is due to multiplet effects, i.e.the exchange <strong>in</strong>teraction between the 3p f<strong>in</strong>al state <strong>and</strong> the3d-states is very large <strong>and</strong> creates the satellites at 7040 eV,split by about 20 eV from the ma<strong>in</strong> peak. The peak splitt<strong>in</strong>g<strong>and</strong> the amount of <strong>in</strong>tensity shifted to this satellite isstrongly dependent on the valence <strong>and</strong> sp<strong>in</strong>-state. This differenceimplies that the ma<strong>in</strong> peak will also have to shift tokeep the center of gravity fixed. It is this multiplet-<strong>in</strong>ducedshift that makes the1s3p XES spectra suitable for valenceselective XAS studies. Note that the1s2p XES is less suitablebecause the 2p3d exchange splitt<strong>in</strong>g is much smaller<strong>and</strong> one expects much smaller shifts <strong>in</strong> the peak maximaof a 1s2p spectrum. The 1s XANES <strong>and</strong> EXAFS weremeasured at various energy positions <strong>and</strong> us<strong>in</strong>g a numericalmethod, the <strong>in</strong>dividual site EXAFS spectra were extractedfrom the experimental data [80]. The pre-edge <strong>and</strong> XANESregions are affected by more complex resonance effectsthat are discussed <strong>in</strong> the review by Glatzel <strong>and</strong> Bergmannelsewhere <strong>in</strong> this issue [49].Instead of us<strong>in</strong>g chemical shifts due to valence differencesof two states, one can make use any variable that caused adifferent decay spectrum, either via electrons, i.e. Auger orvia photons, i.e. XES. In pr<strong>in</strong>ciple, an enormous range ofpossibilities can be used, <strong>in</strong>clud<strong>in</strong>g the use of secondary orcascade decay products, for example, X-<strong>ray</strong> excited opticallum<strong>in</strong>escence (XEOL)signals, etc. Each signal will have itsown characteristic with respect to prob<strong>in</strong>g depth, detectionarea, etc. for example, 1s2p XES will be a local probe,whereas optical signals (XEOL) will emit from a large (r)area around the absorb<strong>in</strong>g atom, etc.A beautiful example, us<strong>in</strong>g soft X-<strong>ray</strong> emission is the detectionof the <strong>in</strong>dependent 1s XAS spectra of N 2 moleculeadsorbed on a nickel surface. By detect<strong>in</strong>g specific decay