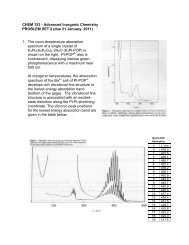
CHEM 153 - Advanced Inorganic Chemistry PROBLEM SET 4 (due ...
CHEM 153 - Advanced Inorganic Chemistry PROBLEM SET 4 (due ...
CHEM 153 - Advanced Inorganic Chemistry PROBLEM SET 4 (due ...
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
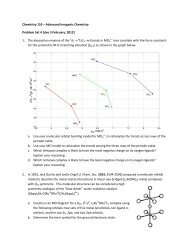
2. Consider a binuclear metal complex constructed fromtwo square-planar ML 4 fragments where the L ligands areσ-donors. There are two limiting conformations in theresulting M 2 L 8 complex: in one the ligands are eclipsed andin the other they are staggered.a. Draw the two conformations of the binuclear metalcomplex and assign each to a symmetry pointgroup.b. Construct an MO diagram for each conformation using the following orbitals: fiveM d orbitals and four L σ orbitals. Assume that M-L σ-bonding is quite strong.c. Assume that ML 4 has a d 4 electron configuration, and that each ligand Lcontributes two σ electrons.1. Predict the preferred ground-state conformation of the corresponding M 2 L 8complex, and give the electronic configuration and term symbol for the groundelectronic state. Determine the metal-metal bond order.2. Identify the spin-allowed electronic transitions involving the d-orbitals,determine the term symbols for the excited states, and predict the relativeenergy ordering of these states.3. Identify the electric-dipole-allowed transitions and determine the polarizationof light that will induce the transition(s).d. Assume that ML 4 has a d 6 electron configuration, and that each ligand Lcontributes two σ electrons.1. Predict the preferred ground-state conformation of the corresponding M 2 L 8complex, and give the electronic configuration and term symbol for the groundelectronic state. Determine the metal-metal bond order.2. Identify the spin-allowed electronic transitions involving the d-orbitals,determine the term symbols for the excited states, and predict the relativeenergy ordering of these states.3. Identify the electric-dipole-allowed transitions and determine the polarizationof light that will induce the transition(s).