#06-5558 Accrd Manual V7 - COLA
#06-5558 Accrd Manual V7 - COLA
#06-5558 Accrd Manual V7 - COLA
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
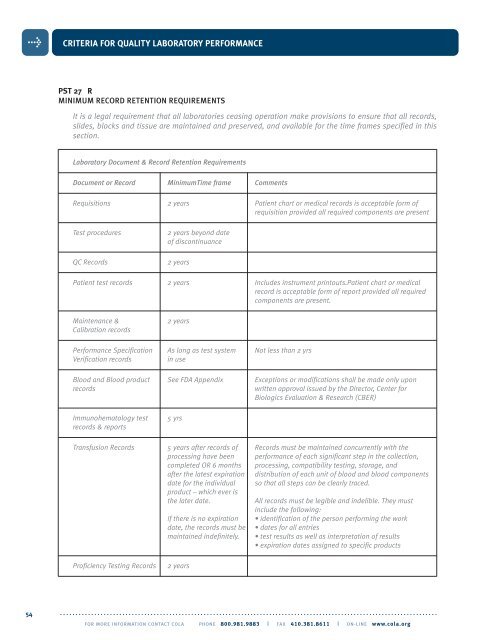
CRITERIA FOR QUALITY LABORATORY PERFORMANCEPST 27 RMINIMUM RECORD RETENTION REQUIREMENTSIt is a legal requirement that all laboratories ceasing operation make provisions to ensure that all records,slides, blocks and tissue are maintained and preserved, and available for the time frames specified in thissection.Laboratory Document & Record Retention RequirementsDocument or Record MinimumTime frame CommentsRequisitions 2 years Patient chart or medical records is acceptable form ofrequisition provided all required components are presentTest proceduresQC Records2 years beyond dateof discontinuance2 yearsPatient test records 2 years Includes instrument printouts.Patient chart or medicalrecord is acceptable form of report provided all requiredcomponents are present.Maintenance &Calibration records2 yearsPerformance Specification As long as test system Not less than 2 yrsVerification recordsin useBlood and Blood product See FDA Appendix Exceptions or modifications shall be made only uponrecordswritten approval issued by the Director, Center forBiologics Evaluation & Research (CBER)Immunohematology testrecords & reports5 yrsTransfusion Records 5 years after records of Records must be maintained concurrently with theprocessing have been performance of each significant step in the collection,completed OR 6 months processing, compatibility testing, storage, andafter the latest expiration distribution of each unit of blood and blood componentsdate for the individual so that all steps can be clearly traced.product – which ever isthe later date.All records must be legible and indelible. They mustinclude the following:If there is no expiration • identification of the person performing the workdate, the records must be • dates for all entriesmaintained indefinitely. • test results as well as interpretation of results• expiration dates assigned to specific productsProficiency Testing Records2 years54. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .FOR MORE INFORMATION CONTACT <strong>COLA</strong> PHONE 800.981.9883 | FAX 410.381.8611 | ON-LINE www.cola.org