#06-5558 Accrd Manual V7 - COLA
#06-5558 Accrd Manual V7 - COLA
#06-5558 Accrd Manual V7 - COLA
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
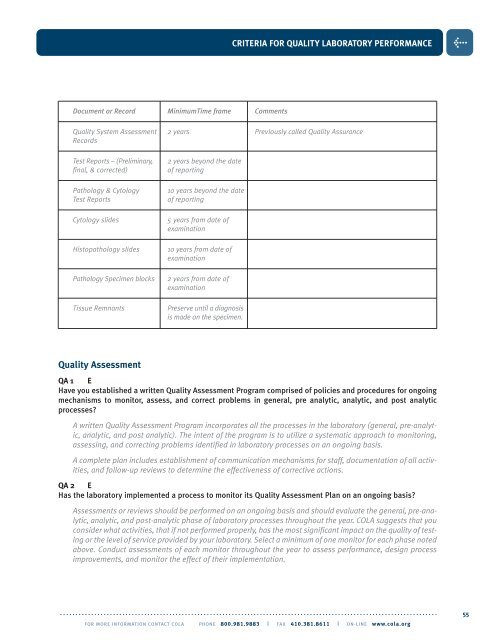
CRITERIA FOR QUALITY LABORATORY PERFORMANCE>Document or Record MinimumTime frame CommentsQuality System Assessment 2 years Previously called Quality AssuranceRecordsTest Reports – (Preliminary,final, & corrected)Pathology & CytologyTest ReportsCytology slidesHistopathology slidesPathology Specimen blocksTissue Remnants2 years beyond the dateof reporting10 years beyond the dateof reporting5 years from date ofexamination10 years from date ofexamination2 years from date ofexaminationPreserve until a diagnosisis made on the specimen.Quality AssessmentQA 1 EHave you established a written Quality Assessment Program comprised of policies and procedures for ongoingmechanisms to monitor, assess, and correct problems in general, pre analytic, analytic, and post analyticprocesses?A written Quality Assessment Program incorporates all the processes in the laboratory (general, pre-analytic,analytic, and post analytic). The intent of the program is to utilize a systematic approach to monitoring,assessing, and correcting problems identified in laboratory processes on an ongoing basis.A complete plan includes establishment of communication mechanisms for staff, documentation of all activities,and follow-up reviews to determine the effectiveness of corrective actions.QA 2 EHas the laboratory implemented a process to monitor its Quality Assessment Plan on an ongoing basis?Assessments or reviews should be performed on an ongoing basis and should evaluate the general, pre-analytic,analytic, and post-analytic phase of laboratory processes throughout the year. <strong>COLA</strong> suggests that youconsider what activities, that if not performed properly, has the most significant impact on the quality of testingor the level of service provided by your laboratory. Select a minimum of one monitor for each phase notedabove. Conduct assessments of each monitor throughout the year to assess performance, design processimprovements, and monitor the effect of their implementation.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .FOR MORE INFORMATION CONTACT <strong>COLA</strong> PHONE 800.981.9883 | FAX 410.381.8611 | ON-LINE www.cola.org55