- Page 1:
(a)(b)Phase 2Phase 12.95 3.00 3.05
- Page 5 and 6:
Detailed Table of ContentsPreface..
- Page 7 and 8:
4C.4 Uncertainty for Mixed Operatio
- Page 10:
8B.1 Theory and Practice . . . . .
- Page 14 and 15:
13. Kinetic Methods .. . . . . . .
- Page 16 and 17:
Appendix 5: Critical Values for the
- Page 18 and 19:
xviii Analytical Chemistry 2.0AOrga
- Page 20 and 21:
xx Analytical Chemistry 2.0original
- Page 22 and 23:
xxii Analytical Chemistry 2.0Steven
- Page 24 and 25:
2 Analytical Chemistry 2.0This quot
- Page 26 and 27:
4 Analytical Chemistry 2.0CH 3NOHHO
- Page 29 and 30:
Chapter 1 Introduction to Analytica
- Page 31 and 32:
Chapter 1 Introduction to Analytica
- Page 33 and 34:
Chapter 1 Introduction to Analytica
- Page 35 and 36:
DRAFTChapter 2Basic Tools ofAnalyti
- Page 37 and 38:
Chapter 2 Basic Tools of Analytical
- Page 39 and 40:
Chapter 2 Basic Tools of Analytical
- Page 41 and 42:
Chapter 2 Basic Tools of Analytical
- Page 43 and 44:
Chapter 2 Basic Tools of Analytical
- Page 45 and 46:
Chapter 2 Basic Tools of Analytical
- Page 47 and 48:
Chapter 2 Basic Tools of Analytical
- Page 49 and 50:
Chapter 2 Basic Tools of Analytical
- Page 51 and 52:
Chapter 2 Basic Tools of Analytical
- Page 53 and 54:
Chapter 2 Basic Tools of Analytical
- Page 55 and 56:
Chapter 2 Basic Tools of Analytical
- Page 57 and 58:
Chapter 2 Basic Tools of Analytical
- Page 59 and 60:
Chapter 2 Basic Tools of Analytical
- Page 61 and 62:
Chapter 2 Basic Tools of Analytical
- Page 63 and 64:
DRAFTChapter 3The Vocabularyof Anal
- Page 65 and 66:
Chapter 3 The Vocabulary of Analyti
- Page 67 and 68:
Chapter 3 The Vocabulary of Analyti
- Page 69 and 70:
Chapter 3 The Vocabulary of Analyti
- Page 72 and 73:
50 Analytical Chemistry 2.0Solving
- Page 74 and 75:
52 Analytical Chemistry 2.0It shoul
- Page 76 and 77:
54 Analytical Chemistry 2.0( S −S
- Page 78 and 79:
56 Analytical Chemistry 2.0StartFig
- Page 80 and 81:
58 Analytical Chemistry 2.0rugged s
- Page 82 and 83:
60 Analytical Chemistry 2.010 ppb g
- Page 84 and 85:
62 Analytical Chemistry 2.0Practice
- Page 86 and 87:
64 Analytical Chemistry 2.0Figure 4
- Page 88 and 89:
66 Analytical Chemistry 2.0Problem
- Page 90 and 91:
68 Analytical Chemistry 2.0The conv
- Page 92 and 93:
70 Analytical Chemistry 2.0be trace
- Page 94 and 95:
72 Analytical Chemistry 2.0Figure 4
- Page 96 and 97:
74 Analytical Chemistry 2.0provided
- Page 98 and 99:
76 Analytical Chemistry 2.0Although
- Page 100 and 101:
78 Analytical Chemistry 2.0Rounding
- Page 102 and 103:
80 Analytical Chemistry 2.0Table 4.
- Page 104 and 105:
82 Analytical Chemistry 2.02 ppm×
- Page 106 and 107:
84 Analytical Chemistry 2.0Table 4.
- Page 108 and 109:
86 Analytical Chemistry 2.0µ= Np =
- Page 110 and 111:
88 Analytical Chemistry 2.0Figure 4
- Page 112 and 113:
90 Analytical Chemistry 2.04D.3 Con
- Page 114 and 115:
92 Analytical Chemistry 2.0196 . ×
- Page 116 and 117:
94 Analytical Chemistry 2.0Figure 4
- Page 118 and 119:
96 Analytical Chemistry 2.0Here is
- Page 120 and 121:
98 Analytical Chemistry 2.0We will
- Page 122 and 123:
100 Analytical Chemistry 2.0The fou
- Page 124 and 125:
102 Analytical Chemistry 2.0We rese
- Page 126 and 127:
104 Analytical Chemistry 2.0Example
- Page 128 and 129:
106 Analytical Chemistry 2.0So l u
- Page 130 and 131:
108 Analytical Chemistry 2.0tsAµ A
- Page 132 and 133:
110 Analytical Chemistry 2.03. 117
- Page 134 and 135:
112 Analytical Chemistry 2.0differe
- Page 136 and 137:
114 Analytical Chemistry 2.0lyte’
- Page 138 and 139:
116 Analytical Chemistry 2.0Table 4
- Page 140 and 141:
118 Analytical Chemistry 2.0dIf s m
- Page 142 and 143:
120 Analytical Chemistry 2.0Once yo
- Page 144 and 145:
122 Analytical Chemistry 2.0obtaini
- Page 146 and 147:
124 Analytical Chemistry 2.0dt-Test
- Page 148 and 149:
126 Analytical Chemistry 2.0> dbino
- Page 150 and 151:
128 Analytical Chemistry 2.0> t.tes
- Page 152 and 153:
130 Analytical Chemistry 2.0(a)Hist
- Page 155 and 156:
Chapter 4 Evaluating Analytical Dat
- Page 157 and 158:
Chapter 4 Evaluating Analytical Dat
- Page 159 and 160:
Chapter 4 Evaluating Analytical Dat
- Page 161 and 162:
Chapter 4 Evaluating Analytical Dat
- Page 163 and 164:
Chapter 4 Evaluating Analytical Dat
- Page 165 and 166:
Chapter 4 Evaluating Analytical Dat
- Page 167 and 168:
Chapter 4 Evaluating Analytical Dat
- Page 169 and 170:
Chapter 4 Evaluating Analytical Dat
- Page 171 and 172:
Chapter 4 Evaluating Analytical Dat
- Page 173 and 174:
Chapter 4 Evaluating Analytical Dat
- Page 175 and 176:
DRAFTChapter 5Standardizing Analyti
- Page 177 and 178:
Chapter 5 Standardizing Analytical
- Page 179 and 180:
Chapter 5 Standardizing Analytical
- Page 181 and 182:
Chapter 5 Standardizing Analytical
- Page 183 and 184:
Chapter 5 Standardizing Analytical
- Page 185 and 186:
Chapter 5 Standardizing Analytical
- Page 187 and 188:
Chapter 5 Standardizing Analytical
- Page 189 and 190:
Chapter 5 Standardizing Analytical
- Page 191 and 192:
Chapter 5 Standardizing Analytical
- Page 194 and 195:
172 Analytical Chemistry 2.05D.1 Li
- Page 196 and 197:
174 Analytical Chemistry 2.0Fi n d
- Page 198 and 199:
176 Analytical Chemistry 2.0where y
- Page 200 and 201:
178 Analytical Chemistry 2.0Minimiz
- Page 202 and 203:
180 Analytical Chemistry 2.0605040S
- Page 204 and 205:
182 Analytical Chemistry 2.0Practic
- Page 206 and 207:
184 Analytical Chemistry 2.0( 6 6 1
- Page 208 and 209:
186 Analytical Chemistry 2.0Check o
- Page 210 and 211:
188 Analytical Chemistry 2.0amount
- Page 212 and 213:
190 Analytical Chemistry 2.0y108642
- Page 214 and 215:
192 Analytical Chemistry 2.00.60.4R
- Page 216 and 217:
194 Analytical Chemistry 2.0> model
- Page 218 and 219:
196 Analytical Chemistry 2.0> inver
- Page 220 and 221:
198 Analytical Chemistry 2.05IProbl
- Page 222 and 223:
200 Analytical Chemistry 2.0(a) Use
- Page 224 and 225:
202 Analytical Chemistry 2.0data se
- Page 226 and 227:
204 Analytical Chemistry 2.00.1478x
- Page 228 and 229:
206 Analytical Chemistry 2.0The sta
- Page 230 and 231:
208 Analytical Chemistry 2.0> libra
- Page 232 and 233:
210 Analytical Chemistry 2.0Napoleo
- Page 234 and 235:
212 Analytical Chemistry 2.0Equatio
- Page 236 and 237:
214 Analytical Chemistry 2.0Rxn1: A
- Page 238 and 239:
216 Analytical Chemistry 2.0A weak
- Page 240 and 241:
218 Analytical Chemistry 2.0Dissoci
- Page 242 and 243:
220 Analytical Chemistry 2.0When fi
- Page 244 and 245:
222 Analytical Chemistry 2.0Example
- Page 246 and 247:
224 Analytical Chemistry 2.0ln(x) =
- Page 248 and 249:
226 Analytical Chemistry 2.0So what
- Page 250 and 251:
228 Analytical Chemistry 2.0One of
- Page 252 and 253:
230 Analytical Chemistry 2.0more ba
- Page 254 and 255:
232 Analytical Chemistry 2.0less li
- Page 256 and 257:
234 Analytical Chemistry 2.0[ Zn(NH
- Page 258 and 259:
236 Analytical Chemistry 2.0When we
- Page 260 and 261:
238 Analytical Chemistry 2.0the ass
- Page 262 and 263:
240 Analytical Chemistry 2.0You may
- Page 264 and 265:
242 Analytical Chemistry 2.0Step 3:
- Page 266 and 267:
244 Analytical Chemistry 2.0pHOH 2
- Page 268 and 269:
246 Analytical Chemistry 2.0[ HO ]K
- Page 270 and 271:
248 Analytical Chemistry 2.0C NH3=
- Page 272 and 273:
250 Analytical Chemistry 2.0Because
- Page 274 and 275:
252 Analytical Chemistry 2.0We can
- Page 276 and 277:
254 Analytical Chemistry 2.0The 1mM
- Page 278 and 279:
256 Analytical Chemistry 2.0In calc
- Page 280 and 281:
258 Analytical Chemistry 2.0Several
- Page 282 and 283:
260 Analytical Chemistry 2.06JUsing
- Page 284 and 285:
262 Analytical Chemistry 2.0From th
- Page 286 and 287:
264 Analytical Chemistry 2.0For exa
- Page 288 and 289:
266 Analytical Chemistry 2.0(a) pH
- Page 290 and 291:
268 Analytical Chemistry 2.06MChapt
- Page 292 and 293:
270 Analytical Chemistry 2.0These r
- Page 294 and 295:
272 Analytical Chemistry 2.015. Cal
- Page 296 and 297:
274 Analytical Chemistry 2.0AgBr()
- Page 298 and 299:
276 Analytical Chemistry 2.0Equilib
- Page 300 and 301:
278 Analytical Chemistry 2.0These t
- Page 302 and 303:
280 Analytical Chemistry 2.0Adding
- Page 304 and 305:
282 Analytical Chemistry 2.0[OH - ]
- Page 306 and 307:
284 Analytical Chemistry 2.0
- Page 308 and 309:
286 Analytical Chemistry 2.0Figure
- Page 310 and 311:
288 Analytical Chemistry 2.0There a
- Page 312 and 313:
290 Analytical Chemistry 2.0Appendi
- Page 314 and 315:
292 Analytical Chemistry 2.0-1.0 -0
- Page 316 and 317:
294 Analytical Chemistry 2.0For exa
- Page 318 and 319:
296 Analytical Chemistry 2.0A sampl
- Page 320 and 321:
298 Analytical Chemistry 2.0Practic
- Page 322 and 323:
300 Analytical Chemistry 2.02of tim
- Page 324 and 325:
302 Analytical Chemistry 2.0capcapw
- Page 326 and 327:
304 Analytical Chemistry 2.0include
- Page 328 and 329:
306 Analytical Chemistry 2.0cable t
- Page 330 and 331:
308 Analytical Chemistry 2.0size an
- Page 332 and 333:
310 Analytical Chemistry 2.0(a)(b)p
- Page 334 and 335:
312 Analytical Chemistry 2.0K × C
- Page 336 and 337:
314 Analytical Chemistry 2.0leaves
- Page 338 and 339:
316 Analytical Chemistry 2.0(a) (b)
- Page 340 and 341:
318 Analytical Chemistry 2.0Table 7
- Page 342 and 343:
320 Analytical Chemistry 2.0Table 7
- Page 344 and 345:
322 Analytical Chemistry 2.0(a)(b)t
- Page 346 and 347:
324 Analytical Chemistry 2.0Separat
- Page 348 and 349:
326 Analytical Chemistry 2.0μL dro
- Page 350 and 351:
328 Analytical Chemistry 2.0samplet
- Page 352 and 353:
330 Analytical Chemistry 2.0tograph
- Page 354 and 355:
332 Analytical Chemistry 2.0D =( mo
- Page 356 and 357:
334 Analytical Chemistry 2.0Extract
- Page 358 and 359:
336 Analytical Chemistry 2.0+K [ HO
- Page 360 and 361:
338 Analytical Chemistry 2.0Extract
- Page 362 and 363:
340 Analytical Chemistry 2.0grab sa
- Page 364 and 365:
342 Analytical Chemistry 2.0Time (h
- Page 366 and 367:
344 Analytical Chemistry 2.0Nominal
- Page 368 and 369:
346 Analytical Chemistry 2.0for Cu
- Page 370 and 371:
348 Analytical Chemistry 2.0organic
- Page 372 and 373:
350 Analytical Chemistry 2.0To obta
- Page 374 and 375:
352 Analytical Chemistry 2.0Fe 2+p(
- Page 376 and 377:
354 Analytical Chemistry 2.0
- Page 378 and 379:
356 Analytical Chemistry 2.0Method
- Page 380 and 381:
358 Analytical Chemistry 2.0Other e
- Page 382 and 383:
360 Analytical Chemistry 2.0The pre
- Page 384 and 385:
362 Analytical Chemistry 2.0(a) (b)
- Page 386 and 387:
364 Analytical Chemistry 2.0A super
- Page 388 and 389:
366 Analytical Chemistry 2.0primary
- Page 390 and 391:
368 Analytical Chemistry 2.0(a)(b)(
- Page 392 and 393:
370 Analytical Chemistry 2.0Fritted
- Page 394 and 395:
372 Analytical Chemistry 2.0Each mo
- Page 396 and 397:
374 Analytical Chemistry 2.0Table 8
- Page 398 and 399:
376 Analytical Chemistry 2.0Practic
- Page 400 and 401:
378 Analytical Chemistry 2.0Althoug
- Page 402 and 403:
380 Analytical Chemistry 2.0of ±0.
- Page 404 and 405:
382 Analytical Chemistry 2.0thermog
- Page 406 and 407:
384 Analytical Chemistry 2.0(a)bala
- Page 408 and 409: 386 Analytical Chemistry 2.03. Why
- Page 410 and 411: 388 Analytical Chemistry 2.0So l u
- Page 412 and 413: 390 Analytical Chemistry 2.0Alterna
- Page 414 and 415: 392 Analytical Chemistry 2.0(a)(b)(
- Page 416 and 417: 394 Analytical Chemistry 2.0Example
- Page 418 and 419: 396 Analytical Chemistry 2.06. Mixi
- Page 420 and 421: 398 Analytical Chemistry 2.0(a) If
- Page 422 and 423: 400 Analytical Chemistry 2.0nese, a
- Page 424 and 425: 402 Analytical Chemistry 2.032. The
- Page 426 and 427: 404 Analytical Chemistry 2.0Mass (g
- Page 428 and 429: 406 Analytical Chemistry 2.0Practic
- Page 430 and 431: 408 Analytical Chemistry 2.0Of the
- Page 432 and 433: 410 Analytical Chemistry 2.0
- Page 434 and 435: 412 Analytical Chemistry 2.0We are
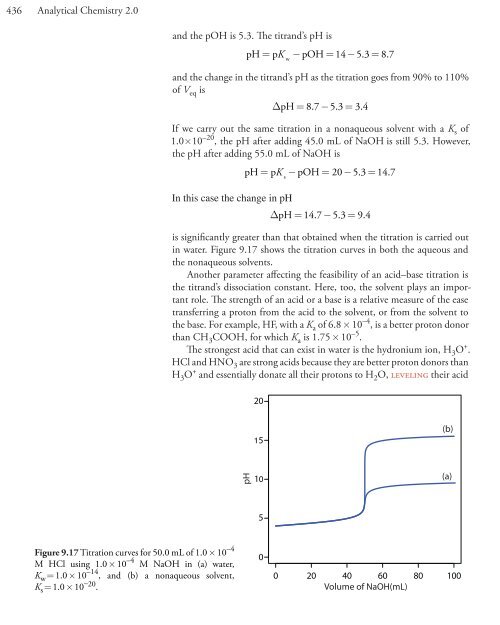
- Page 436 and 437: 414 Analytical Chemistry 2.0This is
- Page 438 and 439: 416 Analytical Chemistry 2.0Tempera
- Page 440 and 441: 418 Analytical Chemistry 2.0time an
- Page 442 and 443: 420 Analytical Chemistry 2.0141210p
- Page 444 and 445: 422 Analytical Chemistry 2.0Kb−[
- Page 446 and 447: 424 Analytical Chemistry 2.014 (a)1
- Page 448 and 449: 426 Analytical Chemistry 2.014 (a)1
- Page 450 and 451: 428 Analytical Chemistry 2.0141210M
- Page 452 and 453: 430 Analytical Chemistry 2.0You may
- Page 454 and 455: 432 Analytical Chemistry 2.0Suppose
- Page 456 and 457: 434 Analytical Chemistry 2.0Excess
- Page 460 and 461: 438 Analytical Chemistry 2.0below r
- Page 462 and 463: 440 Analytical Chemistry 2.0Table 9
- Page 464 and 465: 442 Analytical Chemistry 2.01412(a)
- Page 466 and 467: 444 Analytical Chemistry 2.0Table 9
- Page 468 and 469: 446 Analytical Chemistry 2.0192.13
- Page 470 and 471: 448 Analytical Chemistry 2.00. 1183
- Page 472 and 473: 450 Analytical Chemistry 2.0Practic
- Page 474 and 475: 452 Analytical Chemistry 2.00. 1005
- Page 476 and 477: 454 Analytical Chemistry 2.0A secon
- Page 478 and 479: 456 Analytical Chemistry 2.0microbu
- Page 480 and 481: 458 Analytical Chemistry 2.0There a
- Page 482 and 483: 460 Analytical Chemistry 2.0pH10.24
- Page 484 and 485: 462 Analytical Chemistry 2.0NH 4 +
- Page 486 and 487: 464 Analytical Chemistry 2.0initial
- Page 488 and 489: 466 Analytical Chemistry 2.0Practic
- Page 490 and 491: 468 Analytical Chemistry 2.0Note th
- Page 492 and 493: 470 Analytical Chemistry 2.0(a) 10(
- Page 494 and 495: 472 Analytical Chemistry 2.0The bes
- Page 496 and 497: 474 Analytical Chemistry 2.0In o r
- Page 498 and 499: 476 Analytical Chemistry 2.0Finally
- Page 500 and 501: 478 Analytical Chemistry 2.0108(a)p
- Page 502 and 503: 480 Analytical Chemistry 2.0Althoug
- Page 504 and 505: 482 Analytical Chemistry 2.0Table 9
- Page 506 and 507: 484 Analytical Chemistry 2.01.6(a)(
- Page 508 and 509:
486 Analytical Chemistry 2.0So l u
- Page 510 and 511:
488 Analytical Chemistry 2.0For sim
- Page 512 and 513:
490 Analytical Chemistry 2.0The bes
- Page 514 and 515:
492 Analytical Chemistry 2.0of the
- Page 516 and 517:
494 Analytical Chemistry 2.0Potassi
- Page 518 and 519:
496 Analytical Chemistry 2.0oxidize
- Page 520 and 521:
498 Analytical Chemistry 2.0with I
- Page 522 and 523:
500 Analytical Chemistry 2.0Practic
- Page 524 and 525:
502 Analytical Chemistry 2.0Subtrac
- Page 526 and 527:
504 Analytical Chemistry 2.0Step 3:
- Page 528 and 529:
506 Analytical Chemistry 2.010(a)10
- Page 530 and 531:
508 Analytical Chemistry 2.0Table 9
- Page 532 and 533:
510 Analytical Chemistry 2.0Example
- Page 534 and 535:
512 Analytical Chemistry 2.0continu
- Page 536 and 537:
514 Analytical Chemistry 2.0Some of
- Page 538 and 539:
516 Analytical Chemistry 2.0Some of
- Page 540 and 541:
518 Analytical Chemistry 2.00.0316
- Page 542 and 543:
520 Analytical Chemistry 2.0(g) The
- Page 544 and 545:
522 Analytical Chemistry 2.0Some of
- Page 546 and 547:
524 Analytical Chemistry 2.043. Cal
- Page 548 and 549:
526 Analytical Chemistry 2.0and tit
- Page 550 and 551:
528 Analytical Chemistry 2.09ISolut
- Page 552 and 553:
530 Analytical Chemistry 2.0pHFigur
- Page 554 and 555:
532 Analytical Chemistry 2.0Practic
- Page 556 and 557:
534 Analytical Chemistry 2.0−32+(
- Page 558 and 559:
536 Analytical Chemistry 2.0Practic
- Page 560 and 561:
538 Analytical Chemistry 2.0E (V)0.
- Page 562 and 563:
540 Analytical Chemistry 2.0( 0. 04
- Page 564 and 565:
542 Analytical Chemistry 2.0or a pC
- Page 566 and 567:
544 Analytical Chemistry 2.0Figure
- Page 568 and 569:
546 Analytical Chemistry 2.0Example
- Page 570 and 571:
548 Analytical Chemistry 2.0Table 1
- Page 572 and 573:
550 Analytical Chemistry 2.0Table 1
- Page 574 and 575:
552 Analytical Chemistry 2.0radiant
- Page 576 and 577:
554 Analytical Chemistry 2.0Monochr
- Page 578 and 579:
556 Analytical Chemistry 2.0dynodeh
- Page 580 and 581:
558 Analytical Chemistry 2.0Why doe
- Page 582 and 583:
560 Analytical Chemistry 2.01.21.0a
- Page 584 and 585:
562 Analytical Chemistry 2.0(a)P 0s
- Page 586 and 587:
564 Analytical Chemistry 2.0Example
- Page 588 and 589:
566 Analytical Chemistry 2.0For a m
- Page 590 and 591:
568 Analytical Chemistry 2.0sources
- Page 592 and 593:
570 Analytical Chemistry 2.0Figure
- Page 594 and 595:
572 Analytical Chemistry 2.0of fibe
- Page 596 and 597:
574 Analytical Chemistry 2.0fromsou
- Page 598 and 599:
576 Analytical Chemistry 2.0Table 1
- Page 600 and 601:
578 Analytical Chemistry 2.0H 2 NO
- Page 602 and 603:
580 Analytical Chemistry 2.0in any
- Page 604 and 605:
582 Analytical Chemistry 2.0mg Fe/L
- Page 606 and 607:
584 Analytical Chemistry 2.0Practic
- Page 608 and 609:
586 Analytical Chemistry 2.0A mix /
- Page 610 and 611:
588 Analytical Chemistry 2.0IR spec
- Page 612 and 613:
590 Analytical Chemistry 2.01.0mole
- Page 614 and 615:
592 Analytical Chemistry 2.0from co
- Page 616 and 617:
594 Analytical Chemistry 2.0Proceed
- Page 618 and 619:
596 Analytical Chemistry 2.0Ac c u
- Page 620 and 621:
598 Analytical Chemistry 2.00% Tsam
- Page 622 and 623:
600 Analytical Chemistry 2.0(a)burn
- Page 624 and 625:
602 Analytical Chemistry 2.0This is
- Page 626 and 627:
604 Analytical Chemistry 2.0Because
- Page 628 and 629:
606 Analytical Chemistry 2.0See Cha
- Page 630 and 631:
608 Analytical Chemistry 2.0Most in
- Page 632 and 633:
610 Analytical Chemistry 2.0Example
- Page 634 and 635:
612 Analytical Chemistry 2.0per ana
- Page 636 and 637:
614 Analytical Chemistry 2.0S 2vrvr
- Page 638 and 639:
616 Analytical Chemistry 2.0quinine
- Page 640 and 641:
618 Analytical Chemistry 2.0z-axisy
- Page 642 and 643:
620 Analytical Chemistry 2.0by immo
- Page 644 and 645:
622 Analytical Chemistry 2.0The bes
- Page 646 and 647:
624 Analytical Chemistry 2.0[quinin
- Page 648 and 649:
626 Analytical Chemistry 2.0Se l e
- Page 650 and 651:
628 Analytical Chemistry 2.06000 K8
- Page 652 and 653:
630 Analytical Chemistry 2.0Plasmas
- Page 654 and 655:
632 Analytical Chemistry 2.0sodium
- Page 656 and 657:
634 Analytical Chemistry 2.0See Sec
- Page 658 and 659:
636 Analytical Chemistry 2.0(a)sour
- Page 660 and 661:
638 Analytical Chemistry 2.0TI=IT0T
- Page 662 and 663:
640 Analytical Chemistry 2.0The bes
- Page 664 and 665:
642 Analytical Chemistry 2.0Example
- Page 666 and 667:
644 Analytical Chemistry 2.0of anal
- Page 668 and 669:
646 Analytical Chemistry 2.08. A se
- Page 670 and 671:
648 Analytical Chemistry 2.0Crystal
- Page 672 and 673:
650 Analytical Chemistry 2.0A sampl
- Page 674 and 675:
652 Analytical Chemistry 2.0P total
- Page 676 and 677:
654 Analytical Chemistry 2.0absorba
- Page 678 and 679:
656 Analytical Chemistry 2.08 .0 10
- Page 680 and 681:
658 Analytical Chemistry 2.0solutio
- Page 682 and 683:
660 Analytical Chemistry 2.01.04 1.
- Page 684 and 685:
662 Analytical Chemistry 2.043. Sel
- Page 686 and 687:
664 Analytical Chemistry 2.0C Cu0.1
- Page 688:
666 Analytical Chemistry 2.0A−AHI