Guidelines for the Use of RFID Technology in Transfusion Medicine
Guidelines for the Use of RFID Technology in Transfusion Medicine
Guidelines for the Use of RFID Technology in Transfusion Medicine
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
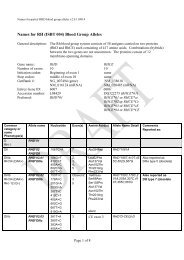
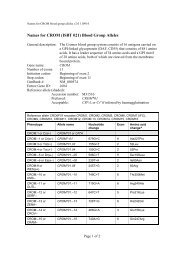
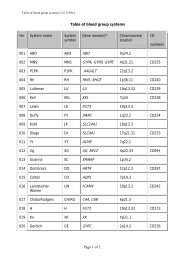
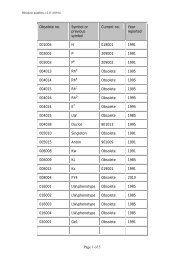
10 Guidel<strong>in</strong>edoes not supply <strong>RFID</strong> tags as part <strong>of</strong> <strong>the</strong> donation set. Insome organizations, it may not be possible to providepatient identity on <strong>the</strong> tag because <strong>of</strong> data security legislation.As each ISBT 128 data structure is uniquely identifiable[1], it may not be necessary to def<strong>in</strong>e an overall fixedtag memory structure but it would probably be more efficient,with respect to IT systems, if a standard structurewere <strong>in</strong> place.Tag Manufacturers<strong>RFID</strong> tag manufacturers encode <strong>the</strong> UID, which is designedto be unique globally, on <strong>the</strong> chip. Part <strong>of</strong> this code is anAFI and <strong>for</strong> transfusion medic<strong>in</strong>e <strong>the</strong> code ‘‘BBH’’ wasrequested from <strong>the</strong> ISO committee. The UID code is unique;<strong>the</strong>re<strong>for</strong>e, it may be <strong>the</strong> only data item on <strong>the</strong> tag that isbe<strong>in</strong>g read dur<strong>in</strong>g rout<strong>in</strong>e process<strong>in</strong>g.Blood Bag ManufacturersISBT128 provides data structures perta<strong>in</strong><strong>in</strong>g to manufacturers<strong>of</strong> blood bags, namely <strong>the</strong> ‘Conta<strong>in</strong>er Manufacturer andCatalogue Number’, <strong>the</strong> ‘Conta<strong>in</strong>er Lot Number’, and <strong>the</strong>‘Expiry Date’. The m<strong>in</strong>imum data elements needed are <strong>the</strong>Catalogue Number and Lot Number [1].It may be possible to go fur<strong>the</strong>r than <strong>the</strong>se standards. Forexample, <strong>the</strong> current lot number relates to a batch <strong>of</strong> manyunits <strong>the</strong>re<strong>for</strong>e, <strong>in</strong> <strong>the</strong> event <strong>of</strong> a recall, <strong>the</strong> number <strong>of</strong> packsimplicated can be high. However, if <strong>the</strong> UID is used <strong>in</strong> <strong>the</strong>manufactur<strong>in</strong>g process, considerable benefits should bega<strong>in</strong>ed <strong>for</strong> all parties as it may be possible to limit <strong>the</strong> scope<strong>of</strong> a recall.Bag manufacturer data should be locked on <strong>the</strong> tag aspart <strong>of</strong> <strong>the</strong> pack manufactur<strong>in</strong>g operation. It should not bepossible to amend this data once written; it should belocked irrevocably.Blood CentersISBT 128 def<strong>in</strong>es data structures <strong>for</strong> use by blood centers[1]. It will be necessary to align <strong>the</strong> data structures with <strong>the</strong>data blocks on <strong>the</strong> tag. Twelve data blocks are reserved <strong>for</strong>use by blood centers. Mandatory data <strong>in</strong>cludes donationnumber, product ID and expiration date ⁄ time, and ABO ⁄ Rh.Donation number should be encoded and locked at <strong>the</strong> earliestopportunity. This may be as soon as <strong>the</strong> donation iscollected and donation number label sets are applied to <strong>the</strong>bag set. Alternatively, this data may be encoded when <strong>the</strong>donor ⁄ donation l<strong>in</strong>k is made on an IT system. O<strong>the</strong>r collectiondata could be encoded and locked at <strong>the</strong> same time.The data blocks reserved <strong>for</strong> product code and expirationdate ⁄ time are rewritable to allow <strong>for</strong> remanufactur<strong>in</strong>g <strong>in</strong><strong>the</strong> same bag, such as irradiation. ABO ⁄ Rh is written onceand locked.Blood center data on <strong>the</strong> <strong>RFID</strong> tag will normally belocked as part <strong>of</strong> <strong>the</strong> shipp<strong>in</strong>g process. However,flexibility will be likely to be needed to allow product<strong>in</strong><strong>for</strong>mation lock<strong>in</strong>g at any time. For example, a bloodcenter could issue a unit <strong>of</strong> red cells subsequently irradiatedby a hospital. In this case, it would be necessary toallow <strong>the</strong> hospital to update <strong>the</strong> product <strong>in</strong><strong>for</strong>mation and,potentially, <strong>the</strong> expiration date. Alternatively, productdata could be locked by <strong>the</strong> blood center. The lock<strong>in</strong>gunder such scenarios is likely to require flexibility at anational level.Because <strong>of</strong> <strong>the</strong> limited tag capacity available, it may benecessary to use a Special Test<strong>in</strong>g data structure related to<strong>the</strong> product be<strong>in</strong>g issued. One <strong>of</strong> <strong>the</strong> limitations <strong>of</strong> currentl<strong>in</strong>ear barcode symbols is that it is difficult to fit specialtest<strong>in</strong>g data with<strong>in</strong> <strong>the</strong> available space. The higher memorycapacity <strong>in</strong> <strong>RFID</strong> tags will provide an alternative solution<strong>for</strong> a long-stand<strong>in</strong>g label space problem.HospitalsHospitals and transfusion services can add data to <strong>the</strong> tagregard<strong>in</strong>g <strong>the</strong> <strong>in</strong>tended recipient. Hemovigilance studiesshow that many serious adverse <strong>in</strong>cidents are because <strong>of</strong>errors that occur at <strong>the</strong> patient <strong>in</strong>terface. This is an areawhere <strong>RFID</strong> can make a significant difference.It must be recognized that application <strong>of</strong> patient data to<strong>the</strong> <strong>RFID</strong>-tag will be subject to national patient privacy anddata security legislation and may not be permissible.Where hospitals can add this <strong>in</strong><strong>for</strong>mation, it will be necessaryto determ<strong>in</strong>e what sort <strong>of</strong> <strong>in</strong><strong>for</strong>mation is required by<strong>the</strong> hospitals <strong>for</strong> positive patient identification. For <strong>the</strong> purposes<strong>of</strong> this guidel<strong>in</strong>e, it has been assumed that 96 byteswould be sufficient.Examples <strong>of</strong> tag structureThe follow<strong>in</strong>g three examples show how product-specificISBT128 data structures could be presented on a tag whileensur<strong>in</strong>g that <strong>the</strong> data is aligned with <strong>the</strong> four-bytelock<strong>in</strong>g blocks (Figs 5–7). With<strong>in</strong> each colored block, <strong>the</strong>symbols use <strong>the</strong> same <strong>for</strong>m as with<strong>in</strong> <strong>the</strong> ISBT 128 specification[1].4.6 DisposalThe destruction <strong>of</strong> passive <strong>RFID</strong> tags can follow standardbiohazard destruction protocols used <strong>for</strong> blood bags (medicalwaste <strong>in</strong>c<strong>in</strong>erators).If active <strong>RFID</strong> tags are used on conta<strong>in</strong>ers, recycl<strong>in</strong>gshould be addressed to con<strong>for</strong>m with exist<strong>in</strong>g recommendations<strong>for</strong> battery recycl<strong>in</strong>g.<strong>RFID</strong> tags do not fall with<strong>in</strong> <strong>the</strong> scope <strong>of</strong> <strong>the</strong> EU directive2002 ⁄ 96 ⁄ EC on Waste Electrical and Electronic Equipment(WEEE) if <strong>the</strong> tag is not attached to o<strong>the</strong>r electronic equipmentwhich falls with<strong>in</strong> <strong>the</strong> scope <strong>of</strong> <strong>the</strong> WEEE directive[19].Ó 2010 The Author(s)Journal compilation Ó 2010 International Society <strong>of</strong> Blood <strong>Transfusion</strong>, Vox Sangu<strong>in</strong>is (2010) 98 (Suppl. 2), 1–24